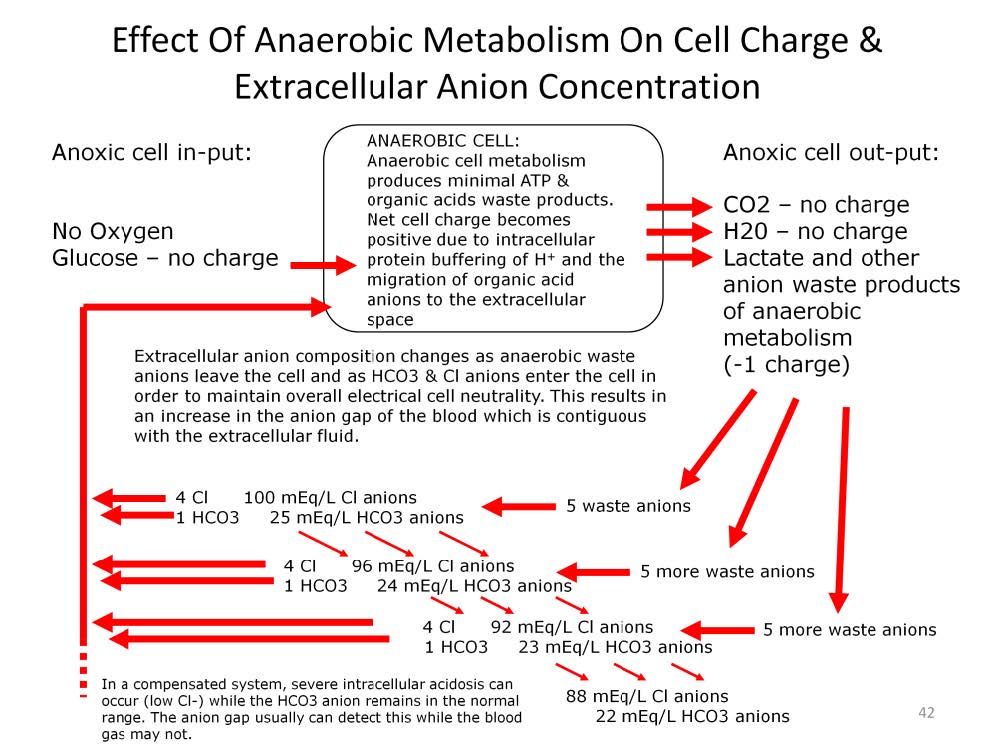
In the anoxic cell, there is no oxygen available. The glucose enters the cell, undergoes anaerobic metabolism with the resultant waste products of water, carbon dioxide and organic acids such as lactic acid. The hydronium cation from the organic acid is chemically complexed to the intracellular protein buffers. However the organic acid anion, mostly lactate, is free to migrate outside of the cell.
In the extracellular fluid the electrolyte composition is very similar and contiguous with the blood serum electrolytes. In this example, there is an extracellular anion concentration that includes 100 mEq/L of the Cl- anion and 25 mEq/L of the HCO3– anion. The cell becomes positively charged by the hydronium cation which becomes chemically complexed to intracellular buffers while the negatively charged lactate ion migrates out of the cell. Subsequently an equivalent number of major extracellular anions must migrate intracellularly in order to maintain overall cell electrical neutrality. This has the effect of changing the composition of the major extracellular anions and disrupts the balance between the cations and anions in the extracellular fluid and blood serum. This disruption is quantified as an increased anion gap (AG); the difference between the total cation charge and the total anion charge.

Perfusion Theory is an educational platform for the Oxygen Pressure Field Theory (OPFT). August Krogh’s theoretical concept of the oxygen pressure field is explained and then applied to clinical applications in perfusion practice.