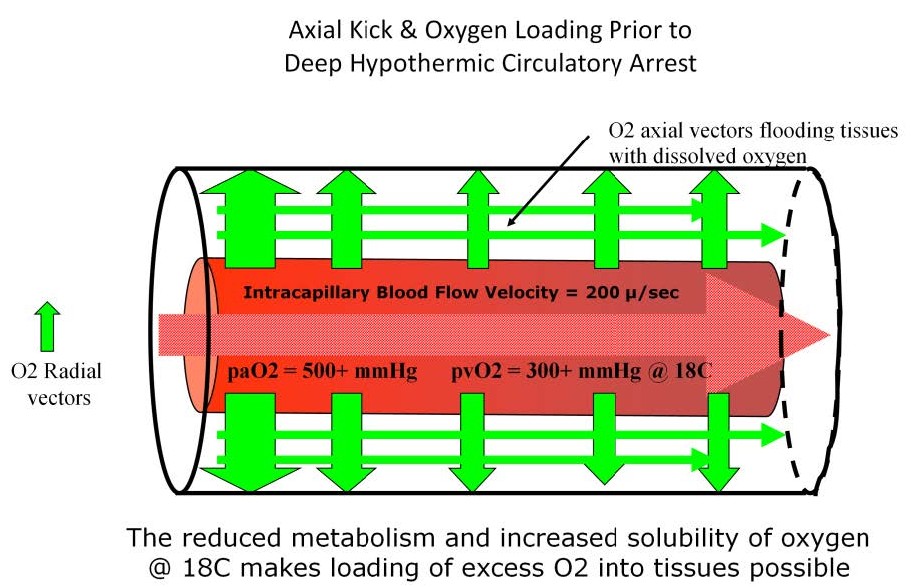
Hyperoxygenation prior to DHCA floods the tissues with dissolved oxygen and extends the safe arrest time before anaerobic metabolism starts.
The technique of deep hypothermic circulatory arrest (DHCA) is a good example of how PCD changes and how those changes can effect perfusion strategy. Patients survive hypothermic arrest procedures because of the reduced metabolic rate and reduced oxygen consumption, but there is a limit to how long this arrest period can last without damage. By stopping blood flow through the capillaries during DHCA, the capillary-to-cylinder ratio infinitely increases so that PCD is essentially zero. With virtually no flowing blood to supply the tissue cylinders, the entire body becomes one large Krogh cylinder containing a potentially huge lethal corner. At 18 °C, cerebral oxygen consumption is only about 20% of normal. Also oxygen is about 2/3rds more soluble (0.05 cc/mmHg/kg of H2O) at 18 °C than at 37 °C (0.03 cc/mmHg/kg of H2O). By taking advantage of the increased solubility of oxygen and the reduced oxygen requirements at 18 °C, dissolved oxygen can be stored in the very tissues where it will be used during the arrest period; not just on the hemoglobin in the blood but in the Krogh cylinders themselves. The tissue cylinders are flooded with oxygen during cooling using a high FiO2 to augment both radial and axial vectors so that all the tissues within the capillary bed are saturated just prior to DHCA. The use of axial vectors to flood a potential lethal corner with oxygen is known as “axial kick”. An increase in the venous pO2 during cooling is indicative of the relative level of tissue oxygen loading. Reaching a venous pO2 greater than of 300 mmHg may be a more important parameter indicating when to initiate DHCA than simply reaching the target temperature or cooling for an arbitrary minimum time period.
The development of neurological pathology after cardiopulmonary bypass with DHCA has been associated with shorter cooling periods. Supposedly a cooling time that is too short causes uneven cooling of the brain tissue even if the target temperature is reached, resulting in brain damage during the arrest period. However an equally plausible explanation is that longer cooling periods allow for increased storage of oxygen in the tissues. By having more oxygen on board prior to DHCA, the tissues can better tolerate extended periods without circulation. An increased level of dissolved oxygen in the tissues would extend the time before anaerobic metabolism begins. Once anaerobic metabolism starts, intracellular pH worsens due to the production of lactic acid. This sets up a situation where re-oxygenation of the tissues can result in reperfusion injury when circulation is restored.

Perfusion Theory is an educational platform for the Oxygen Pressure Field Theory (OPFT). August Krogh’s theoretical concept of the oxygen pressure field is explained and then applied to clinical applications in perfusion practice.