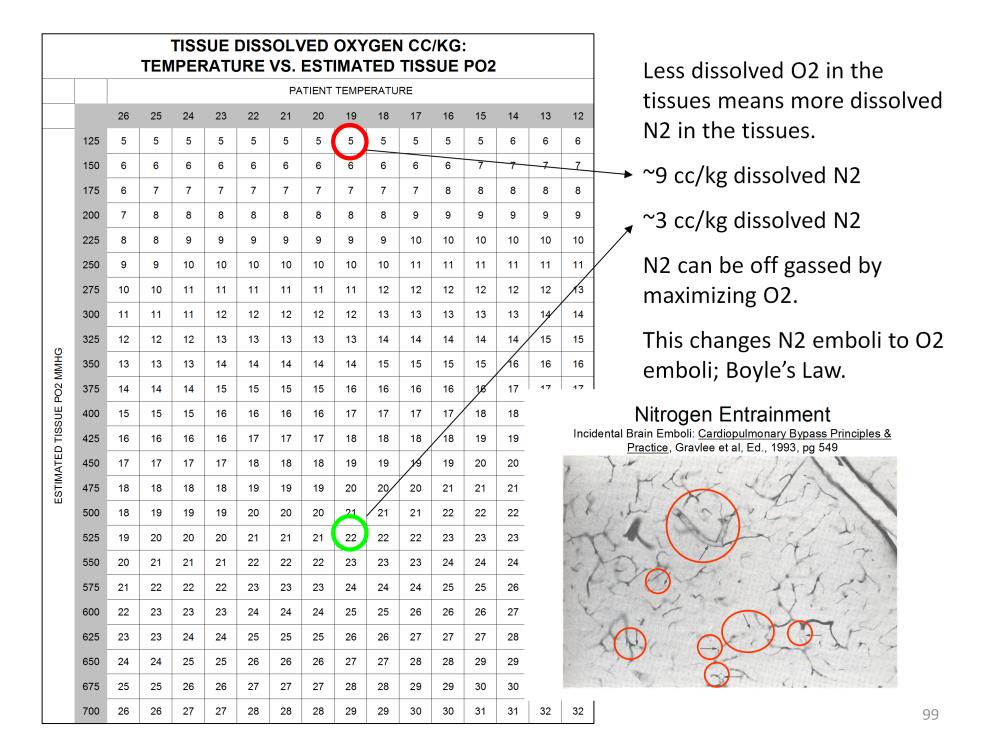
An average tissue pO2 of only 125 mmHg at 18C that translates to 5cc/kg of dissolved oxygen and 9cc/kg of dissolved nitrogen. However if the tissue pO2 is 525 mmHg at 18C that translates to 22cc/kg of dissolved oxygen and only 3cc/kg of dissolved nitrogen.
If less oxygen is loaded into cold tissues, by default, more nitrogen is loaded. Removing this nitrogen during rewarming can be problematic. Rewarming decreases the solubility of nitrogen. Similarly nitrogen becomes less soluble in a diver rising from the depths as the pressure decreases. During the ascent (or rewarming) nitrogen bubbles can form causing ‘the bends’. As rewarming progress, nitrogen bubbles can form in the tissues before it diffuses into the capillaries. The potential also exists for nitrogen bubbles to form within capillaries, potentially blocking the blood flow as depicted in the above photo.

Perfusion Theory is an educational platform for the Oxygen Pressure Field Theory (OPFT). August Krogh’s theoretical concept of the oxygen pressure field is explained and then applied to clinical applications in perfusion practice.