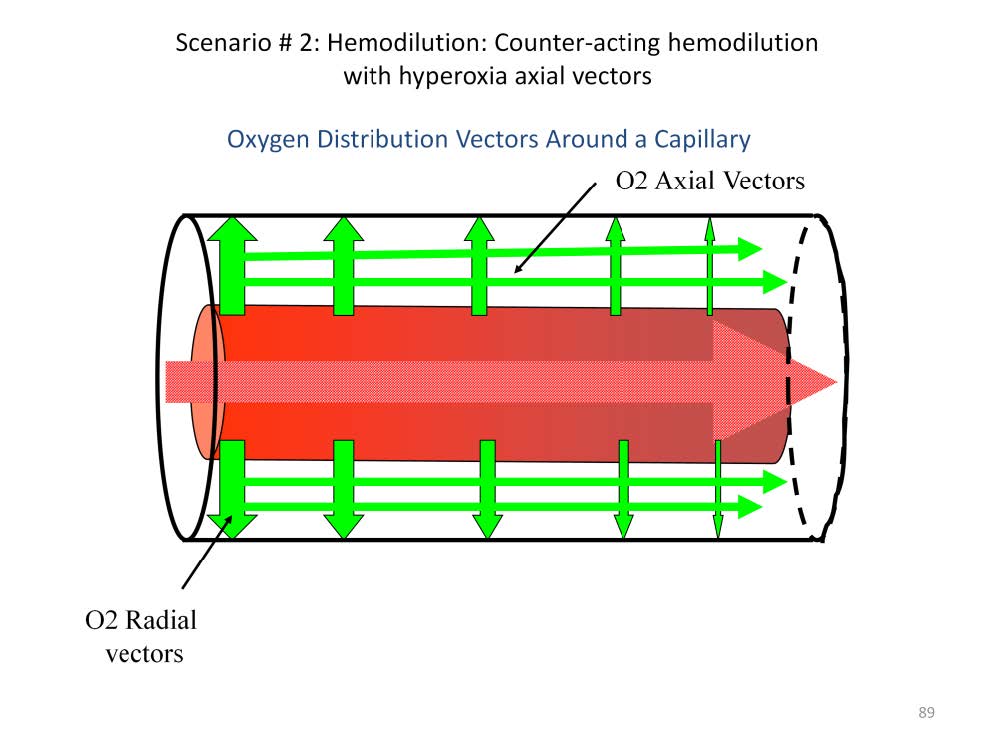
In a healthy patient most of the oxygen that enters the tissues moves along radial gradients (illustrated by the perpendicular arrows) between the blood in the capillary and the tissues. Radial gradients radiate at right angles from the central axis of the capillary. The magnitude of these gradients determine the amount and speed that the oxygen enters the tissues. For example, if the paO2of the blood is 150 mmHg and the tissue pO2 at the arterial end of the cylinder averages 37 mmHg, the radial gradient is 113 mmHg. At the venous end, however, the pvO2 of the blood is only 36 mmHg and the tissue pO2 at the venous end of the cylinder averages 9 mmHg, so the radial gradient is only 27 mmHg. This means that more oxygen will move into the arterial end of the Krogh cylinder at a faster rate than at the venous end of the cylinder. This results in an axial oxygen gradient between the arterial-end-tissue and the venous-end-tissues (illustrated by the narrow, horizontal arrows). Axial gradients run parallel to the axis of the capillary within the substance of the tissue sleeve. It has been calculated that 80% of the oxygen entering the venous end of the Krogh cylinder comes from radial gradients and 20% comes from axial gradients if the intra-capillary blood flow velocity is normal (about 200 μ/second).
The development of metabolic acidosis is often closely tied to hemodilution. The reduced oxygen carrying capacity of blood reduces the effectiveness of a normal cardiac output to deliver adequate amounts of oxygen. During CPB, hemodilution can commonly reduce the hemoglobin concentration in the blood by as much as 50%. And yet, perfusionists do not normally compensate by increasing the cardiac output, as the Fick principal would suggest doing:
Normal oxygen delivery: *DO2 = CO X CaO2
With hemodilution, O2 delivery is reduced by 50%: DO2 / 2 = CO X CaO2 / 2
Hemodilution can be compensated by doubling cardiac output: DO2 = [CO X 2] X CaO2 / 2.
Normally perfusionists do not increase blood flow to compensate for hemodilution. As a result, metabolic acidosis is commonly seen during CPB. Augmenting axial oxygen vectors by increasing the sweep gas FiO2 can compensate for this and often reverse the development of a base deficit during CPB.
*DO2 = oxygen delivery, CO = cardiac output, CaO2 = the content of oxygen in the arterial blood

Perfusion Theory is an educational platform for the Oxygen Pressure Field Theory (OPFT). August Krogh’s theoretical concept of the oxygen pressure field is explained and then applied to clinical applications in perfusion practice.