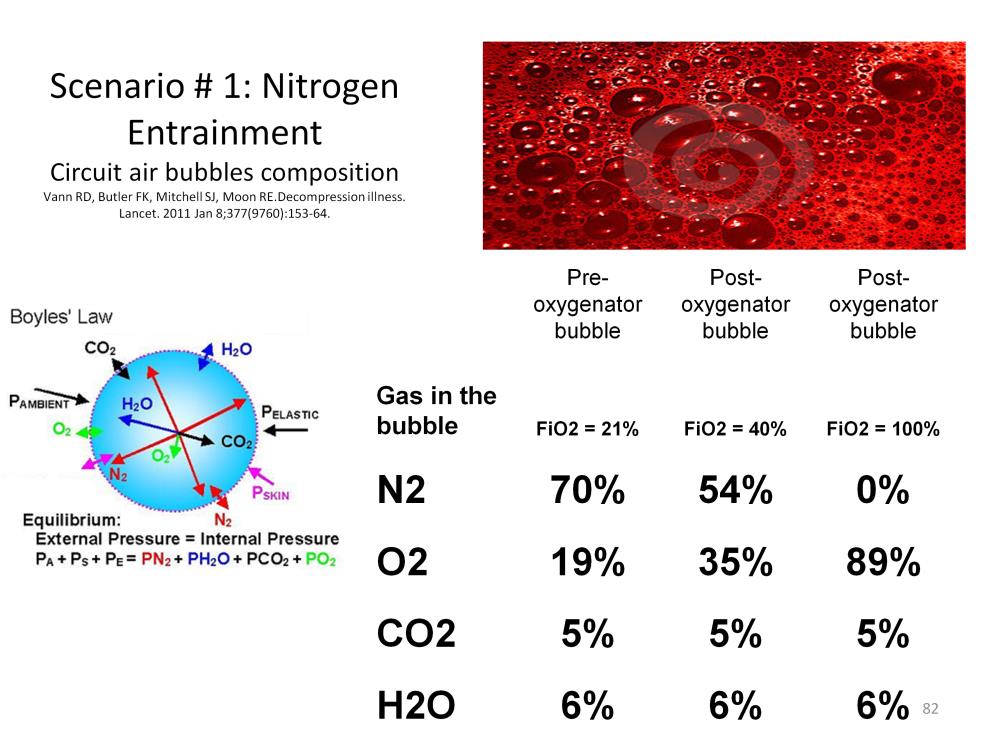
Most bubbles that enter the CPB circuit are initially composed of room air. Based on Boyles’ Law, the bubbles will equilibrate with the dissolved gas in the surrounding fluid. In this case, the surrounding fluid is venous blood which also contains carbon dioxide. Water vapor will also enter the bubble, making the final composition approximately 70% nitrogen, 19% oxygen, 5% carbon dioxide and 6% water vapor. Thus the bubble will be composed primarily of an insoluble and non-absorbable gas; nitrogen.
If the bubble then passes through an oxygenator using a sweep gas FiO2 of 40%, the nitrogen composition of the bubble will decrease to 54% and the oxygen increase to 35%, but the bulk of the bubble will remain a non-absorbable gas.
However if the bubble were to pass through an oxygenator using a sweep gas of 100% oxygen, the nitrogen content of the bubble would decrease to nothing; the bubble being converted to primarily oxygen. Although oxygen is only slightly more soluble than nitrogen, it is a biologically active gas which means that a living system will quickly absorb it and convert it to carbon dioxide. Even though an oxygen GME can obstruct capillary flow, it will be absorbed in a short time with the subsequent restoration of blood flow.
If the situation is such that excessive air is entering the CPB circuit, the perfusionist can combat the danger of non-absorbable nitrogen GME by converting them to oxygen GME. This is easily done by turning the sweep gas FiO2 to 100% oxygen.

Perfusion Theory is an educational platform for the Oxygen Pressure Field Theory (OPFT). August Krogh’s theoretical concept of the oxygen pressure field is explained and then applied to clinical applications in perfusion practice.