The Complacency Trap and Perfusionists Part 1 by Gary Grist RN CCP Emeritus
“Complacency is the lethal enemy of excellence.” Clark Gaither, MD, FAAP, Medical Director of the North Carolina Physicians Health Program.
COMPLACENCY
Complacency is a human failing that is very hard to quantify, but that has not stopped some from trying (1). Yet it is one of the most common causes of mistakes and accidents in business, industry, transportation, the military and even healthcare. No perfusionist would acknowledge that he or she is complacent. But one definition of complacency is being satisfied with how things are going while, at the same time, being oblivious of deficiencies and dangers. In other words, a perfusionist may not be aware that he or she is complacent. Complacency is not carelessness. Carelessness is the absolute disregard of safety. Perfusionists are professionals and definitely not careless.
THE COMPLACENCY TRAP
Being complacent does not necessarily mean that a person is lazy or neglectful, either. Complacency can sneak up on a perfusionist. Indeed, a perfusionist can be highly conscientious and still be complacent. How is that possible? Here is an example. Perfusionists always have a hand crank at the ready should a pump fail, particularly the arterial pump. This is being conscientious. But in cases of an arterial pump failure, this is a poor remedy because hand cranking even a centrifugal pump (which is considerably easier than a roller pump) quickly becomes tiring and saps the attention away from the other tasks and monitoring needed to operate the pump safely. Finding another pump to replace the defective unit and actually replacing it in situ safely and quickly, particularly if no educated help is available, can be extremely difficult. Furthermore, since this is a rare event, this emergency scenario is rarely practiced by clinical programs. It is likely that should the pump fail at a critical moment during the case the patient may be severely injured or killed. Having the hand crank is conscientious, but not thinking of the consequences beyond having to use it is complacency. This is known as a complacency trap; being complacent and not being aware of it. I was caught in such a trap many years ago. And by reading my story, perhaps others will recognize their entrapment as well. (The answer to the pump failure conundrum can be found here (2).)
AUTIOMATION-INDUCED COMPLACENCY
Complacency can be induced when tasks that were previously performed manually are replicated by automation (1). A recent example of this is highlighted in a report from the Automobile Association of America (3). Advanced driver assistance systems (ADAS) such as adaptive speed control and lane-keeping steering assistance are supposed to increase safety and driving comfort. However, drivers who used ADAS frequently became more reliant on them and less reliant on their own driving skills with less focus on the road. The report warns that individual behavioral adaptation of ADAS can result in overreliance and over-trust in the automation features, resulting in more safety-critical events. ADAS make vehicles safer, but in doing so individual operators may become less safe. In other words, drivers can become complacent. So what does this have to do with perfusionists? If we aren’t careful, technological advancements in perfusion can have the same effect on us. I know because it happened to me.
SVO2 MONITORS
I have written about my early years as a perfusionist and detailed the extremes I practiced in assuring my patients were being adequately perfused (4). In the early 1980’s SVO2 monitors were invented and incorporated into the cardiopulmonary bypass (CPB) circuit as an automated, in-line, instantaneous, physiology monitor and safety device. This was a huge step forward for perfusionists. (A step that may be under appreciated by younger perfusionists.) At long last, we had a continuous and instantaneous measurement of an important perfusion parameter; the venous blood oxygen saturation (SVO2). No more waiting 10-20 minutes for venous blood values to be called from the lab, or holding up a red pack of Marlboro or Pall Mall cigarettes next to the venous line to see if the colors matched indicating that the venous saturation was adequate. (The old timers will know what I mean.) Now, should the SVO2 drift out of the accepted range, the perfusionist could make an instantaneous blood flow correction to rectify it.
CONTROLLING PERFUSION PHYSIOLOGY
Back then with the new SVO2 monitoring, the feeling in the perfusion community was that keeping the SVO2 in the 70% +/-5 range was a primary signpost of adequate perfusion. The pump should be run fast enough to achieve this goal. Even if using hypothermia the pump speed could be reduced as long as the SVO2 remained in the target range (a technique I have never condoned). The cardiac index became more of a guideline and not a scrupulously followed regimen. If the mean arterial pressure was low the perfusionist could either speed up the pump or, more commonly, give short acting vasopressors. If the pressure was too high the pump flow could be reduced or gaseous anesthetic administered into the sweep gas as long as the SVO2 remained >65%. If the intermittent blood gas analysis (usually arterial) was acidotic, the perfusionist could give some NaHCO3 (or less commonly THAM) to bring the pH back into line. In other words, just treat the symptom and not the cause of the acidosis. But so long as the SVO2 was acceptable, things were thought to be going well. I believe this was the beginning of “Automation-Induced Complacency” in perfusion (1).
BUBBLERS AND ACUTE RENAL FAILURE
Of course, we did a lot of other things as well to manage perfusion physiology; operate the vent and sucker pumps, manage cardioplegia, change the patient’s temperature using wall water, perform manual charting, perform coronary perfusion, etc. This was in the days of bubble oxygenators. So, sweep gas control was very simple: running carbogen (O2 95% / CO2 5%) gas flow equal to the blood flow. Bubble oxygenators were notoriously damaging to the blood, meaning that their safe use time was quite short; only a few hours. In fact, many surgeons would place a peritoneal dialysis catheter at the start of cases more complicated than a VSD repair knowing that the excessive plasma free hemoglobin generated by the bubbler during long cases was likely to cause acute renal failure in the early post-op period.
EDEMA
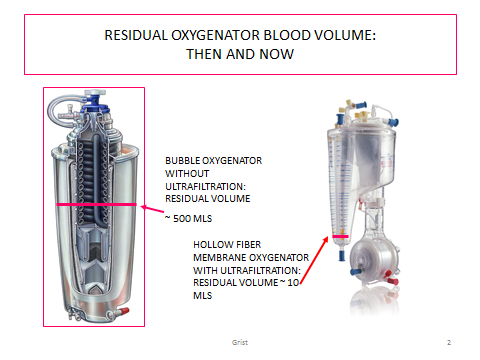
Another common issue with bubblers was that of edema. Two or three liters of edema are hardly visible on an adult, but I worked in pediatrics, and the edema that developed in the operative period was always apparent when the drapes came off. A baby weighing 3.5 kg before surgery would often weigh 4 kg or more immediately after surgery, a 15%+ increase in weight just from water. The child often looked like the Pillsbury Doughboy after surgery and sometimes was covered with petechiae, which can occur when edema ruptures capillaries. If they are visible on the outside, there is probably capillary disruption on the internal organs as well. Ultrafiltrators were not available to help remove excess fluid and infant kidneys are not mature enough to rapidly remove the excess fluid. Some physicians thought that a moderate amount of edema was benign. In fact, the treatment for pulmonary hypertension that prevented weaning from CPB was to preload the right heart with fluid to force it to overcome the pulmonary resistance. Sometimes this worked and sometimes it did not. In the latter case, the child was loaded with inotropes. Either way, the child became more and more edematous. In later years, ultrafiltration in pediatric CPB became a necessity to remove excess fluid and improve the hematocrit (Fig. 1). Later still, I would learn how important fluid balance measurement is during CPB (5).
 |
| Figure 1 |
HYPEROSMOLARITY
Another complacency issue was the osmolarity of my pump prime. These were often blood primes. Since the prime volume was quite large compared to the child’s blood volume, it had a significant impact on changing the blood composition of the patient including blood osmolarity. Banked blood has a very high glucose content which raised the osmolarity above normal. The citrate in the blood necessitated the addition of calcium and the pH was very acidotic requiring the addition of sodium bicarbonate. All this elevated the osmolarity of the prime to 350 mOsm/L or higher. Normal blood osmolarity is 270-300 mOsm/L. Renal damage occurs when a patient’s blood osmolarity reaches 320 and brain damage occurs when it reaches 370. Moreover, by adding more bicarbonate during CPB to treat acidosis I was unknowingly making the osmolarity higher. An elevated osmolarity made diuresis difficult, preventing the patient from getting rid of the edema. Which is the less of two evils, leaving the pH low or making the osmolarity high? That was a question I never asked myself during this time period, because I was doing what I was taught and what many other perfusionists were doing as well; complacency in the raw despite my conscientiousness. In later years I would document the dangers of an elevated osmolarity in babies and children, but that was decades away (6,7).
DOING A GOOD JOB
Oxygenation was never a problem with a bubbler, but I realized that I could never keep a child alive for more than a few hours. There was a lot more to perfusion physiology that I did not understand. But despite this and all the complications, I felt I was doing as good a job as other perfusionists at other programs and the surgeons were happy with our outcomes. I did not realize it but I was trapped in an “Automation-Induced Complacency” trap triggered by the new SVO2 monitors. As long as I kept the SVO2 and pH in acceptable ranges I was doing well. Then ECMO came along.
THE ADVENT OF ECMO
Neonatal ECMO came to my home hospital in 1986, pushed for by the neonatologists. This would be my first experience with membrane oxygenators. And, despite being a pediatric nurse, these were my first encounters with the common and often lethal neonatal respiratory diseases of meconium aspiration, respiratory distress syndrome, group B strep infection, syncytial respiratory virus and persistent fetal circulation. I did not know anything about how to treat these patients. Apparently, the neonatologists did not know much more because they wanted to use a heart/lung pump to keep these patients alive while the disease ran its course. This was not treatment; it was supportive care. I was skeptical that a pump could keep these patients alive and in good shape for the 5-7 days needed, but it was working at the nation’s fourteen other ECMO programs. Maybe the new silicone membrane lungs could work miracles. Anyway, I was willing to try because the alternative was a dead baby.
ONLY 75% SURVIVAL
Several people from my home hospital including myself, physicians and nurses who were to be the back bone of our program attended outside ECMO training programs. Even one heart surgeon came for training. But at that time ECMO was not being considered as a rescue option for “failure to wean from CPB” patients or for dying post-op cardiac patients. (I have described my journey in this regard as well (8).) In our first four years of neonatal ECMO our survival rate was about 75%. This was less than the nationwide figures from the Extracorporeal Life Support Organization (ELSO) for the same four years. Still, most of the ECMO team was happy with our results and anticipated improvement with additional experience. But I was greatly troubled.
THE TROUBLING 25%
The 25% that died distressed me. The physicians around me just seemed to be accepting of these deaths as part of the overall process (physician complacency?). I understood that ECMO was risky, and some patients would not survive. But I was troubled by the causes of these deaths. ECMO patients had lethal complications that were not directly related to their diagnoses. They died of brain hemorrhages, infarct strokes, sepsis unresponsive to antibiotics, unresolved pulmonary edema, cardiac stun, multiple organ failure (kidney, liver, intestinal sloughing), or simply failure to improve. When I queried the neonatologists about this their explanation was either ‘excessive inflammatory response’ or ‘reperfusion injury’ from an earlier hypoxic episode, both of which were aggravated by the pump. That sounded more like an excuse than an explanation. If excessive inflammation was caused by extended exposure to the circuit, why didn’t all the patients have these complications? The same went for reperfusion injury. This was a time when inflammation and reperfusion injury caused by extracorporeal circuits were not well understood.
COMPLACENCY EPIPHANY
Because of these unsettling ECMO deaths, I finally realized that I had been complacent in my approach towards perfusion practice. I had been satisfied with how I was doing things in CPB and felt that any complications were just the hazards that patients must accept for heart surgery and ECMO. I finally realized that there was a lot more to perfusion physiology than a good SVO2, a normal blood pH and an adequate blood pressure. My complacency came to an end in 1990 and I began to seriously look for answers even though I had no idea where to look or what I was seeking.
THE LETHAL CORNER
I finally found the road “less traveled by ” (as articulated by Robert Frost)”and that has made all the difference”. My search for the lethal corner and a new perspective on perfusion physiology has been detailed elsewhere (9). So I won’t reiterate it here. But the new perspective was named the oxygen pressure field theory (OPFT) by August Krogh (1874-1949). I realized it involved a lot more than just oxygenation. There were other associated concepts that even August Krogh never envisioned, but that a 1990’s perfusionist could appreciate (10). As I learned new concepts from OPFT, I applied them to my ECMO patients and tried to teach them to other perfusionists and the many ECMO physicians and nurses with whom I was working (11,12,13,14,15,16,17).
THINGS IMPROVED
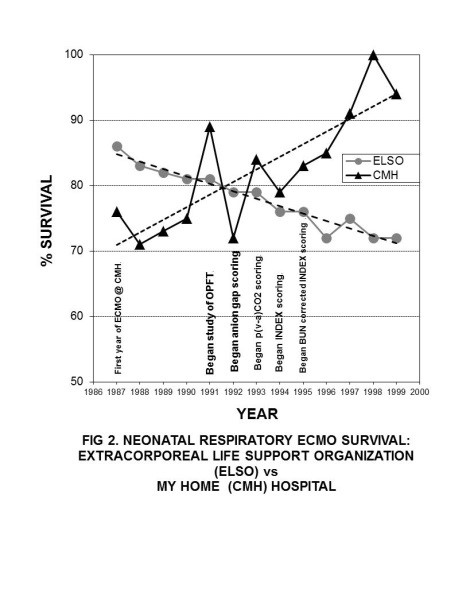
My fellow ECMO team members were correct in that as time passed and our team became more experienced, our patient survival improved (Fig. 2). During this same time period I incorporated things I learned from OPFT and taught other ECMO team members. But the premise that increased experience leads to better outcomes did not seem to apply to the nationwide neonatal ECMO survival rates. Neonatal ECMO survival rates posted by ELSO dropped during this period. I concluded that my efforts were directly increasing our patient survival, lacking nationwide.
End of Part 1
