Should Perfusionists Use a Transfusion Trigger on Cardiopulmonary Bypass?
I recently attended both the New Advances in Blood Management (NABM) and the Society for the Advancement of Blood Management (SABM) in Kansas City in 2009. At both meetings the consensus was that a low hematocrit is bad and a transfusion to increase the hematocrit is even worse, a commonly known clinical paradox (1). At SABM, the emphasis was on medical treatment for anemia (Epogen and iron) and at NABM, the emphasis was on cell salvage and hemoconcentration as a means to improve hematocrit values.
There was also much discussion of how low the hematocrit can be allowed to drop before homologous red cells should be given: the transfusion trigger point. There was no consensus on any specific number. However, an article by DeFoe was cited repeatedly at both meetings as evidence that nadir hematocrit values below 23% on cardiopulmonary bypass (CPB) correlate with increases in morbidity and mortality (2). The data in this article and others provides seemingly strong evidence to justify a red cell transfusion trigger on CPB (3-6). However, the DeFoe article does not recommend using a transfusion trigger in response to a low hematocrit on CPB. Instead, the article suggests reducing perioperative phlebotomy, using CPB circuit volume reduction in patients at risk for excessive hemodilutional anemia, improving management of blood loss and recovery, improving the perioperative management of IV solutions and the reconsideration of autologous and homologous transfusion practice.
The connection between nadir hematocrit on CPB and poor myocardial function is described as plausible but not proven in the DeFoe article even though there is a strong correlation with morbidity and mortality. In considering the risk of death only, patients with hematocrits ≥ 25% had 2% mortality. Patients with hematocrits ≤ 19% had 4% mortality. If 19% is used as a trigger point for transfusion on CPB, the number needed to treat to reduce the mortality by 2% (from 4% to 2%) would be at least 90 out of every 100 low hematocrit patients. This means that more than 90% of the low hematocrit patients would be receiving unnecessary transfusions to achieve a relatively small improvement in mortality.
Red blood cells are important. However, the most important variable is adequate perfusion. Patients with good cardiopulmonary function can tolerate low hematocrit values well. Patients with poor cardiopulmonary function will not do well even with a normalized hematocrit. (7,8) As ‘perfusionists’, perfusion is our specialty and there are ways to optimize perfusion during CPB before resorting to a transfusion trigger.
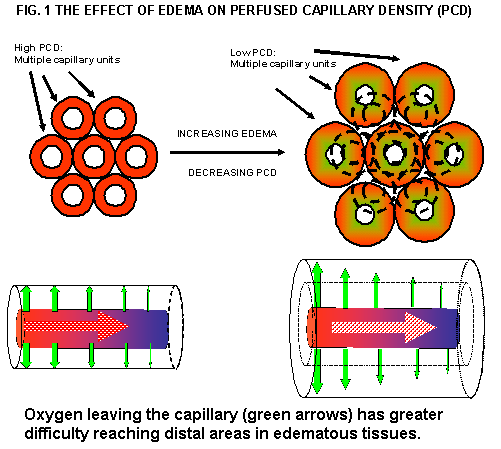
A low hematocrit on CPB does not necessarily correlate to an insufficient red blood cell mass. Low CPB hematocrit values are a reflection of iatrogenic hemodilution of the blood, which leads to edema, which increases morbidity and mortality as seen in the DeFoe article and in postoperative patients in general (9). It is possible that the link between nadir hematocrit on CPB and worse outcomes may be incidental. The actual culprit may be edema caused by iatrogenic hemodilution. This is because edema results in poor tissue oxygenation (10). Edema pushes the capillaries further apart, effectively reducing perfused capillary density, hindering the transfer of dissolved oxygen from the capillary to the tissues furthest from the capillary-tissue interface. See Fig. 1.
Edema is evidence of poor perfusion and is therefore the responsibility of the perfusionist. Edema can be reduced and even prevented by the careful application of continuous ultrafiltration during cardiopulmonary bypass (11). Take, for example, an adult patient entering the OR with 5 kg of excess weight due to edema from congestive heart failure, who then receives 2 liters of IV solutions during the course of the surgery. The seven liters of excess fluid can be removed during CPB. If the procedure lasts 100 minutes, a skilled perfusionist using a 70 milliliter per minute continuous ultrafiltration rate will be able to remove all excess fluid without reducing the circulating volume. If this is then followed by a period of modified ultrafiltration, much of the red blood cell mass will be restored to the patient’s circulation and postoperative blood loss reduced (12,13).
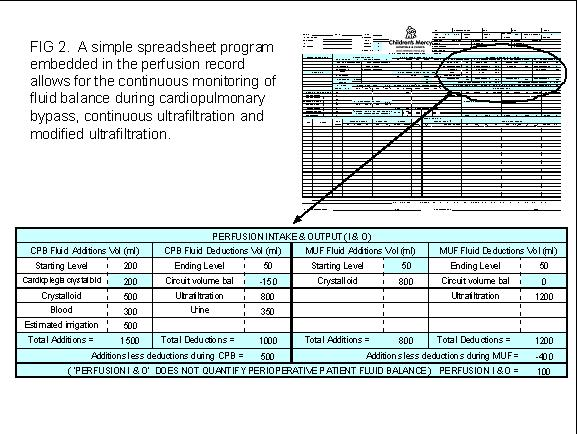
The fluid balance can be easily monitored by utilizing a home made spreadsheet program that calculates fluid balance when additions and subtractions are made to the circuit during the procedure. See Fig. 2.
The meticulous use of ultrafiltration and fluid balance awareness on the part of the perfusionist results in less edematous patients who have less morbidity and mortality in the postoperative period (9). This is confirmed by our own program at The Children’s Mercy Hospitals and Clinics in Kansas City, Missouri, which is primarily a pediatric program. Unpublished data shows that 80% of our patients wean from CPB with negative fluid balances. The remaining 20%, who wean with positive fluid balances have twice the mortality rate of negative fluid balance patients. (This data has since been published*.) This is not due to the perfusionists’ lack of trying to achieve a neutral or negative fluid balance. Positive fluid balance patients tend to be on CPB for longer periods, indicating the need for a more complex repair. They also have increased length of stay, indicating increased morbidity. The failure to achieve a negative fluid balance is most likely the indication of a heart that is higher on the Starling curve; requiring more preload to wean from CPB and probably having less functional reserve in the postoperative period.
Circuit volume reduction, retrograde autologous priming (RAP) and continuous ultrafiltration to reduce hemodilution and remove excess fluid are useful tools, but they may not increase the hemoglobin concentration significantly during CPB. Also, RAP and circuit volume modification may not be available to all perfusionists at all times. The problem then becomes how to improve oxygenation with the limited red blood cell mass available during CPB.
Hyperoxia does not greatly increase oxygen carrying capacity from a quantitative aspect, but from a qualitative aspect it does have benefits in hemodiluted patients. The use of hyperoxia at 400 mmHg is equivalent to increasing the hematocrit by 2% (14). Hyperoxia can also substantially improve tissue oxygenation without increasing the oxygen carrying capacity (15-17). This is done by increasing oxygen distribution vectors in the tissue around individual capillaries (10). The microcirculatory abnormalities associated with hyperoxia in the presence of a normal hematocrit concentration are not encountered in hemodiluted surgical patients and, when used during periods of hemodilution, hyperoxia reduces the need for transfusion and improves tissue oxygenation in all types of surgical patients (18). Hyperoxia may also provide some protection against infection, although this aspect is highly controversial (9).
Some perfusionists avoid hyperoxia at all costs. This is a dogma that has outlived its usefulness, particularly when the avoidance of transfusion is a higher priority. The use of normoxia to limit some undefined and incalculable threat of oxygen toxicity or reperfusion injury in a patient with an abnormally low hematocrit is a misplaced precaution. In no other acute care setting would a critical care specialist limit oxygenation to a patient with critically low hematocrit values. It is true that hyperoxia can be detrimental. The lungs are particularly sensitive to elevated oxygen administration by a ventilator, but the pulmonary damage from hyperoxia requires days to develop. During CPB the lungs are unventilated and their vascular system bypassed, so pulmonary damage from hyperoxia is a non sequitur. Unless the patient has ongoing cyanosis or ischemia that affects other organ systems, the use of hyperoxia during the short period on CPB will not cause lipid peroxidation or reduce antioxidant reserve capacity (19).
In conclusion, the use of a specific transfusion trigger on CPB should not be a consideration. Unless the patient becomes uncontrollably hypoxic or acidotic on CPB, the need for a transfusion should only be considered when all other options have been exhausted or the surgeons intervene with their own preferences.
*Grist G, Whittaker C, Merrigan K, Fenton J, Worrall E, O’Brien J, Lofland G. The Correlation Of Fluid Balance Changes On Cardiopulmonary Bypass To Mortality In Pediatric And Congenital Heart Surgery Patients. J Extra Corpor Technol, Dec, 2011;43(4):215–226