Searching for the Lethal Corner By Gary Grist RN CCP Emeritus
In November of 1990, I was searching the library for some readings pertaining to the perfusion profession. Having been a perfusionist since 1968, I knew all the pertinent facts relating to cardiopulmonary physiology and extracorporeal support. But I was still troubled. In my early years, despite providing oxygenation and good perfusion using my cardiopulmonary bypass (CPB) pump, there were still some disturbing complications after CPB in a few patients. I wondered why patients had “pump head” (disturbed cognition) or acute respiratory distress syndrome (ARDS) after surgery. Why did some patients die of organ failure while most patients did well? The universal fall back explanation was that inflammatory response to the pump caused many of the complications. The patient’s own physiologic weaknesses contributed as well. Improvements in circuits, priming, and technique have ameliorated many of those complications, but not removed them entirely. I grudgingly accepted the inflammatory explanation for many years until extracorporeal membrane oxygenation (ECMO) came along.
We started our ECMO program in 1986. Most ECMO patients survived the extended exposure to an extracorporeal circuit (many days or weeks). So, why didn’t the inflammatory response injure or kill them? It did not make sense. The standard explanation was that most patients could tolerate and even become used to the inflammatory response if exposed to circuits for an extended time. The 20% who died probably could not tolerate it for unknown reasons. That sounded more like rationalization rather than a cause for the complications.
Another thing that did not make sense to me was that some ECMO patients who seemingly were not as sick as others died from an unexpected complication. At the same time, other ECMO patients who were critically ill and required emergent ECMO implementation did well and recovered without severe complications.
Another mystery was that most of those patients who died had a lethal complication not directly related to their pulmonary or cardiac disease. Brain bleeds and brain infarctions were very common as was organ failure or simply failure to improve. The usual explanation was that their shock and/or hypoxia before ECMO had caused the damage. However, that did not always make sense because many patients did not experience severe hypoxia or shock before ECMO. They were often placed on ECMO because the ventilator settings or the need for high doses of support medications were potentially hazardous. Other patients who were placed on ECMO emergently due to severe shock or hypoxia, failing the ventilator and medications, did well and recovered.
The physicians that I worked with felt that each patient was different and that there was no way to predict the outcome with any certainty. I did not believe that. Human beings are complicated biological machines, but machines nonetheless. And problems with machines are always predictable. That is why preventative maintenance is performed; we know what is going to break before it breaks and why it is going to break. That is why, despite all our knowledge about cardiopulmonary physiology, I felt that doctors and perfusionists had missed something very important, some, as yet, unknown concepts or principles.
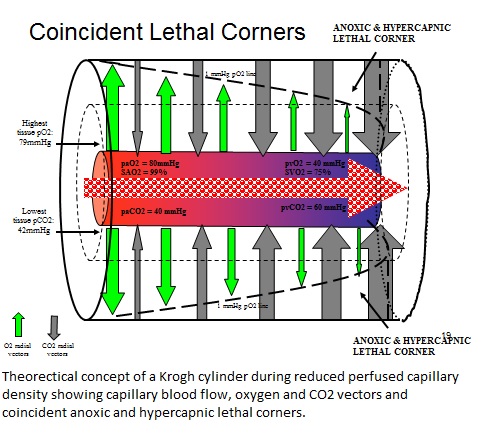
So as I wandered through the library’s stacks that November evening I selected a few books and journals at random for some “light” reading. I opened one journal to an article by Popel (1) that discussed how oxygen was distributed at the capillary level. There were terms that were completely new to me despite my many years of studying cardiopulmonary physiology: the oxygen pressure field, the Krogh cylinder, perfused capillary density, radial and axial oxygen vectors, and the lethal corner. “What is this stuff?” I asked myself. So I followed the references and was introduced to Krogh, Kreuzer, Lubbers, Lund, and Schumacker (2-7).
This was a new concept to me; the oxygen pressure field theory (OPFT). OPFT refers to the partial pressure of oxygen in the field of tissue surrounding individual capillaries.Perhaps everyone else already knew about this concept and they just forgot to tell me! So I went to experienced perfusionists and doctors that I worked with and asked them. They had never heard of OPFT either.
At that time our ECMO program was about four years old and had done about 120 patients with about 75% survival. But I was still nagged by the deaths of the 30 others. As I explained earlier, there was no rational explanation for the seemingly random deaths. Could OPFT solve the mystery? I was in the perfect spot to answer this question. I was the chief perfusionist in an active pediatric ECMO program. Each child on ECMO was closely followed with every blood and physiologic test pertinent to critical care during extracorporeal support. These tests were repeated at regular intervals to monitor progress and safety. All I had to do was to closely observe these tests, keeping in mind that I was trying to discern the oxygen pressure field (if it existed) and detect the lethal corner (a portent of death) if it developed. But where was I to start? What new perspective could I use?
I explained all this to the surgeon I was working with then and asked him for guidance. He looked as if I had stunned him with a ball peen hammer blow to the forehead. He said, “Don’t ask me! Go ask a REAL doctor!” So I went to ask Stanley Hellerstein MD. Stan was a nephrologist who, in the absence of a hematologist at the hospital, had been caring for all the sickle cell (SC) children. He was the smartest doctor I ever knew. I asked Stan what number or parameter could I look at to quantify how sick a cardiopulmonary (CP) patient was. He told me, in his experience, if the CP patient could maintain a normal anion gap (AG), they would most likely survive. So, he thought I should study the patient’s AG before and during ECMO.
As I reviewed the labs on the first 120 ECMO patients, I found that patients with normal AGs at the beginning of ECMO had about a 90% chance of surviving. Of the expired patients, half of them had high AGs while half still had normal AGs. So, an elevated AG had good positive predictive value for death. However, a normal AG had poor negative predictive value for survival. Nonetheless, this was an important first step in discerning the oxygen pressure field and searching for the lethal corner.
I had concluded that the elevated AG was a reflection of the conversion of tissues to anaerobic oxygenation resulting in the production of organic acids like lactic acid. I would later find that for each milliequivalent per liter (mEq/L) that the AG was above the upper limit of normal, mortality would increase by an additional 10%. Patients with normal AG value had a 10% mortality. Patients with an AG of 5 mEq/L above normal had a 50% mortality. And those unfortunate patients with an AG 10 mEq/L above normal had 100% mortality. In essence, the AG was telling me how big the lethal corner was. I would later refer to this as an ‘anoxic lethal corner’ for reasons described below.
Despite finding this lethal corner using the AG, there was still something important missing. I still had dead patients with normal AG values. My focus had been on studying the effects of anaerobic metabolism due to the lack of oxygen. But the second piece of the puzzle had nothing to do with oxygenation.
In 1992 I came across an article by Johnson and Weil describing how the carbon dioxide (CO2) gradient between the arterial and venous blood gases could be used to predict mortality in cardiac patients (8). I was taught to use venous blood gases (VBGs) early on in my career. I had always insisted on drawing both arterial and venous gases on ECMO patients and my CPB patients. But I had not considered that an elevated venous pCO2 (pvCO2) was an indication of intercellular CO2 retention, which caused a detrimental pH change in the cells, stopping their normal metabolic processes.
In reviewing those expired ECMO patients with normal AG values, I found that they had elevated pvCO2 values. This was the other puzzle piece I was looking for. I noticed that for every 1 mmHg that the CO2 gradient was above normal, the mortality increased by 10%. Patients with a CO2 gradient of 7 mmHg had about a 10% mortality and those with a 17 mmHg gradient had 100% mortality. This, then, was another type of lethal corner, not caused by oxygen deprivation, but by intracellular CO2 accumulation; a hypercapnic lethal corner.
Since the AG scale for mortality and the CO2 gradient scale of mortality were about the same (10% increase for each unit increase above normal) I determined that the AG and CO2 gradient scores could be added together as an index score. An elevated index score accounted for 95% of the expired patients. The remaining 5% whose death could not be explained probably died as a result of a lethal anatomy or some unknown inborn error of metabolism.
I concluded that, when patients with a lethal corner were placed on ECMO and suddenly reperfused, they suffered a reperfusion injury that led to the most common lethal complications. Much of my data was published in 2009 and 2010, 19 years after discovering August Krogh’s Oxygen Pressure Field Theory (9, 10). This article does not have the scope to list the many details of what I found in my search for the lethal corner. Those can be seen on my web site, perfusiontheory.com.