Oxygen is a highly reactive molecule; it rots rubber, rusts iron, supports combustion and, in biological systems, is the source of reactive oxygen species (ROS). The most common of these include peroxides, superoxides, hypochlorous acid and hydroxyl radicals. Small amounts of these substances are needed for specific cellular process. However too many can result is significant cell damage because they contain unpaired electrons that can alter normal cell metabolism.
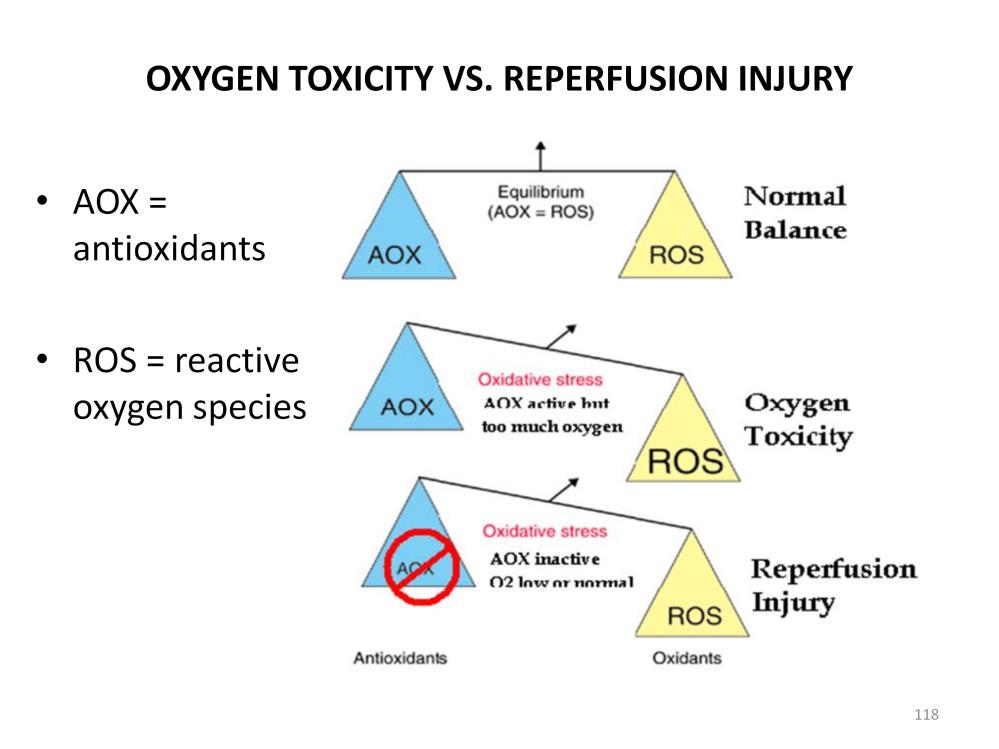
Antioxidants (AOX) are biological firemen that control the production of ROS. The most common include peroxidases, superoxide dismutase, catalase and glutathione. Vitamins such as C & E are coenzymes that aid AOX in their function. Many of the AOX are enzymes that function best when the pH remains within normal physiologic limits (pH > 7.20). Normally ROS production is balanced by AOX function. However ROS can accumulate when the amount of oxygen entering the organism exceeds the functional capacity of the AOX. This is known as oxygen toxicity. Oxygen toxicity occurs even though the AOX are functioning normally and tissue perfusion is unimpaired. It usually requires many hours or days to develop. A common medical situation involving oxygen toxicity is the extended use of high levels of oxygen on the airway and lung tissues. Typically breathing 100% for more than 7 days will result in significant lung damage from oxygen toxicity.
On the other hand, reperfusion injury occurs only after a period of ischemia caused by poor perfusion or hypoxia during which time tissues become anoxic and acidotic. The acidosis stops the normal function of AOX. This is followed by the sudden reintroduction of oxygen when normal perfusion is reestablished. Until normal intracellular pH is restored ROS production is uncontrolled, resulting in significant tissue damage. Unlike oxygen toxicity, the level of oxygen needed to cause damage is not great and the damage occurs immediately upon reperfusion and continues to evolve until normal intracellular pH is restored. The restoration of normal intracellular pH can take many hours, depending on the magnitude of the acidosis, during which time the ROS continue to cause damage.

Perfusion Theory is an educational platform for the Oxygen Pressure Field Theory (OPFT). August Krogh’s theoretical concept of the oxygen pressure field is explained and then applied to clinical applications in perfusion practice.