How Ultrafiltration And MUF During Cardiopulmonary Bypass Really Work!
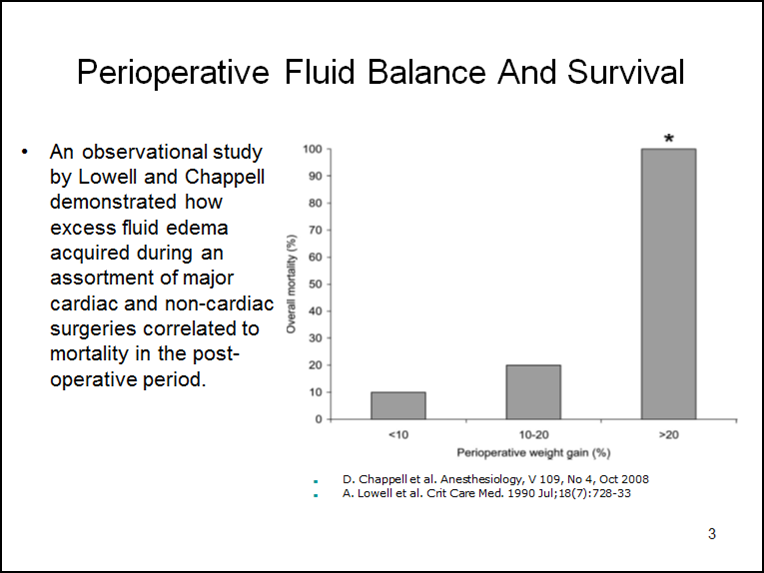
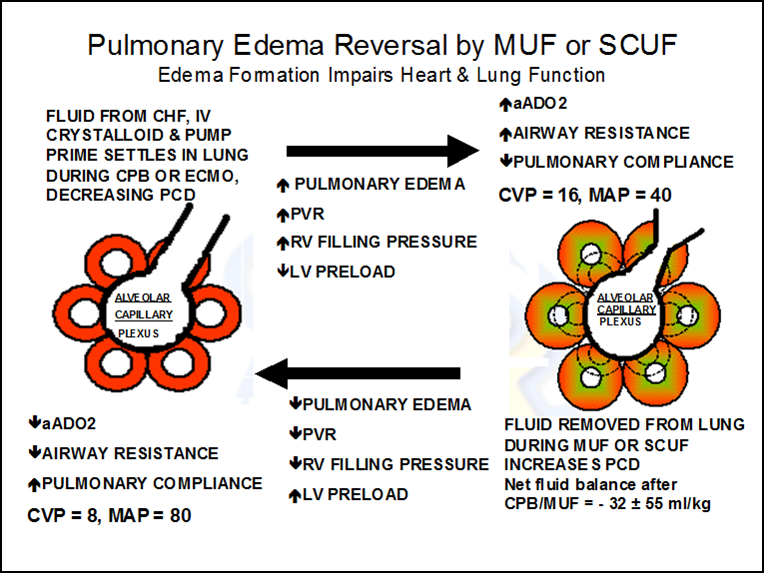
The mechanical separation of capillaries by edema decreases perfused capillary density which disrupts the oxygen pressure field. This can impact most of the soft tissues and organ systems in several ways and is frequently associated with organ failure. For example, the post CPB heart may lack strength and endurance, even though it is well perfused, due to a reduction in tissue oxygen concentration caused by edema. Simultaneously, in the pulmonary system, edema pushes the capillaries away from lung alveoli, causing a reduction in gas exchange. Pulmonary vascular resistance increases as well, impairing blood flow which reduces RV output. This is followed by a reduced LV preload and a further fall in cardiac output. Fluid administration to increase RV preload may provide only temporary improvement in cardiac output while simultaneously worsening the tissue edema. The airway may also be compromised from reduced pulmonary compliance, increased airway resistance and increased oxygen requirements to achieve adequate arterial oxygen saturation. A compensating increase in ventilation positive pressures can further reduce cardiac output. As a potential remedy for all this, removing fluid acquired before and during CPB with conventional ultrafiltration (CUF) and modified ultrafiltration (MUF) may reduce the systemic and pulmonary edema by targeting the corresponding capillary beds. A perfusionist using this strategy can wean most pediatric patients from CPB with negative fluid balances and less mortality than patients who have a positive fluid balance and require additional fluid resuscitation to wean from CPB (1). An observational study by Lowell and published again by Chappell (see below) demonstrates how excess fluid edema acquired during an assortment of major cardiac and non-cardiac surgeries correlates to mortality in the post-operative period (2,3).
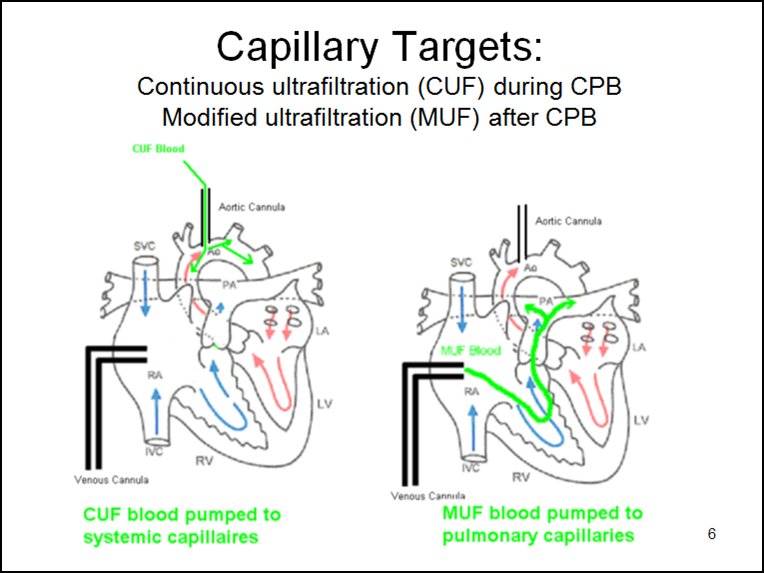
Capillary targets (see below) are important in understanding CUF and MUF. During CPB, CUF sends hemoconcentrated blood into the systemic circulation via the aorta. This tends to remove fluid from the systemic circulatory tissues and reduces overall edema. After CPB, MUF sends hemoconcentrated blood directly into the lungs via the right atrium. This tends to remove fluid from the pulmonary system.
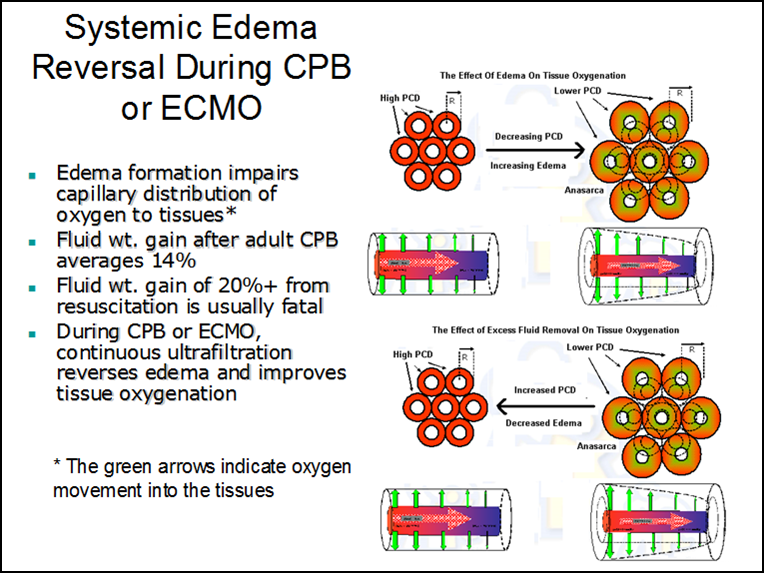
Many critical cardiopulmonary patients require extensive fluid resuscitation at some point during their treatment and will often develop massive edema (anasarca). Severe edema or anasarca pushes the capillaries apart and reduces perfused capillary density (PCD). The result is reduced tissue oxygen concentration and the potential development of an anoxic lethal corner. This mechanical separation decreases PCD which disrupts the oxygen pressure field (the radius of the Krogh cylinders increases). Systemic edema can impact most of the soft tissues and organ systems and is frequently associated with progressive organ failure. This organ failure might be caused by a lethal corner that forms as a result of the disrupted oxygen pressure field even though the capillaries are open. An edematous heart, even though it is well perfused, may lack strength and endurance caused by the decreased tissue oxygen concentration. Aggressive diuresis or ultrafiltration can reduce edema and bring capillaries closer together, restoring the oxygen pressure field to a more survivable configuration (see below).
Edema in the pulmonary system can also result in a decreased PCD that moves the capillaries away from gas exchange near the lung alveoli and increases the vascular resistance to blood flow. This commonly occurs during cardiopulmonary bypass when the lungs do not receive adequate perfusion.
Interstitial edema increases pulmonary vascular resistance and right ventricular filling pressures. This is followed by a reduced left ventricular preload and subsequent fall in cardiac output. Commonly, the treatment is to administer additional fluid to boost right heart output. This may provide temporary improvement in the cardiac output, but the additional fluid can worsen the edema as well.
The airway is also compromised by reduced pulmonary compliance, increased airway resistance and increased oxygen requirements to achieve an adequate arterial oxygen saturation. Combating the edema with ultrafiltration and diuresis can reduce the pulmonary edema and restore pulmonary circulation and function to normal (see below).
Oncotic pressure (colloid osmotic pressure, COP) is exerted across a permeable membrane by the larger molecules in plasma, mostly proteins. The higher COP in blood pulls water from the extravascular space across the capillary membranes and into the circulatory system. These larger molecules remain in the circulatory system and do not cross the capillary membrane.
Osmotic pressure is exerted by smaller molecules like glucose and salt ions in plasma. These also pull water from the extravascular space and into the circulatory system, but eventually the molecules cross the membrane and equilibrate with fluid on both sides of the membrane.
In infants and small children, blood passing through the hemoconcentrator during MUF develops a high COP as excess fluid is removed from it. The ratio of the blood passing through the hemoconcentrator to the total venous return to the right atrium is approximately 1:5 (MUF blood flow: venous return to the lungs = 100 ml/min MUF flow : 500 ml/min VR to the lungs). The high oncotic MUF blood combines with right heart venous return blood raising the oncotic pressure of the blood going directly to the lungs. This higher oncotic blood drags water from lung tissue, improving pulmonary and hemodynamic function. Typically there is a 5-10% hematocrit increase and the clotting factors are concentrated and more effective at reducing bleeding. The average fluid balance after CUF and MUF during CPB is a negative 32 mls/kg. The drop in the CVP from 16 to 8 mmHg illustrated in the diagram above does not only respresent this fluid loss, but more importantly, an improvement in right heart function. With improved right heart function, there is better preload to the left heart. This results in improved cardiac output and an increase in blood pressure from 40 to 80 mmHg. This is an unusual circumstance wherein removal of a subsantial amount of fluid (-32 mls/kg) will improve the patient’s hemodynamics. Patients with a positive fluid balance have twice the mortality rate as negative balance patients (1).
In adults, the ratio of the blood passing through the hemoconcentrator to the total venous return to the right atrium is approximately 1:20 (MUF blood flow: venous return to the lungs = 200 ml/min MUF flow : 4000 ml/min VR to the lungs). The high oncotic MUF blood is diluted by the high volume of right heart blood. The result is that blood with a relatively low COP goes to the lungs. So there is no significant oncotic pressure differential to drag water from lung tissue. The pulmonary benefits are negligible. The typical hematocrit increase is only 1- 4%. And the clotting factors may be minimally concentrated which may or may not reduce bleeding.
Fortunately the pulmonary effect of MUF can be improved in adults. For example, assume that after CPB the patient’s osmolarity is 300 mosmoles/L. Further assume that the residual circuit volume of 1 liter is the same osmolarity (300 mosmoles/L). By mixing 50 mEq/L NaHCO3 or 2 gm/L mannitol into the circuit volume, the osmolarity of residual circuit volume increases to 400 mosmoles/L. Upon commencing MUF, the MUF blood flow of 200 mls/min (400 mosmoles/L) combined with the venous return of 4000 mls/min patient blood (300 mosmoles/L) will increase the osmolarity of the blood passing through the right heart to the lungs from 300 to 305 mosmoles/L. The osmotic drag will remove 67 mls/min of fluid from lung tissue. A total of 335 mls can be removed from the lung tissue in 5 minutes of MUF, thus improving pulmonary and hemodynamic function.
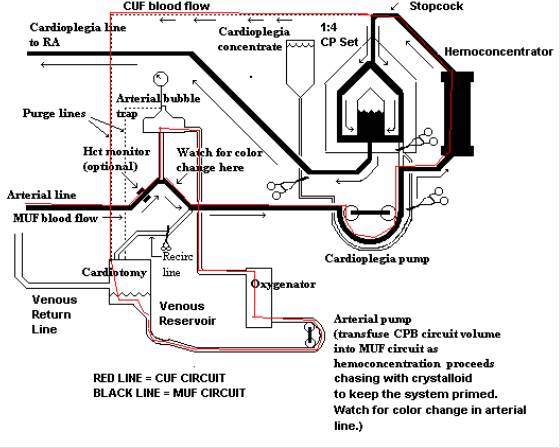
Below is a diagram of the MUF circuit I used.

Great piece on the benefits of MUF. If one did not employ your suggested strategy of adding mannitol or NaHCO3 to the residual pump volume, i would be keen to know your thoughts on when the benefits of MUF (ie the patient size) begin to make the procedure of sufficiently reduced benefit to warrant it unworthy.
In our paediatric practice we vary between MUFing all comers, to MUFing up to 20kg- dependent upon the individual perfusionist.
Of interest I notice that MUF was added to the STS guidelines for adult blood management.
Thank you for your succinct and instructional posting.
Please describe your cannulation and protocol for performing post-CPB MUF technique on adults. And, if possible list any finding associated with adult MUF.
Thank you,
Bob
Bob-
Thank you for your kind comments.
As far as cannulation, the only thing really required is a luer lock connector between the venous cannula and the venous line.
Our UF unit is placed in parallel to the cardioplegia HE in the CP circuit. The CP pump operates both the CP system and the UF. I will try to e-mail a schematic of our system in a separate message.
To start the procedure the venous line is clamped. The CP line it attached to the venous cannula at the luer lock port. The venous line is emptied into the VR. (We use VAVD. So if we need to go back on CPB quickly, the VAVD is started and then the venous line is unclamped.) During MUF the CP pump runs slowly (100-200 mls/min) and aspirates blood from the usual CP port on the oxygenator which draws its blood backwards out of the arterial line and arterial cannula that is still in the AO.
The CP pump pushes blood thru the UF unit. As fluid is removed by the UF unit the arterial pump slowly pumps the residual circuit volume into the MUF circuit. The residual volume is chased by crystalloid until the circuit is clear. This keeps the system primed should it be necessary to reinstitute CPB.
I hope this explains it. Average MUF duration time is 5-10 minutes.
Below is a good article about MUF in adults.
Luciani GB1, Menon T, Vecchi B, Auriemma S, Mazzucco A. Modified ultrafiltration reduces morbidity after adult cardiac operations: a prospective, randomized clinical trial. Circulation. 2001 Sep 18;104(12 Suppl 1):I253-9.
Gary
Nice information,but I want to now what is more useful A-V MUF or V-A MUF??
balwant-
If by A-V MUF you mean aspirating from the aorta and reinfusing through the right atrium (with V-A MUF being just the opposite) then A-V MUF is better for one primary reason. Both methods will concentrate the remaining pump volume and remove excess fluid from the patient’s blood. However A-V MUF specifically targets the lungs and pulmonary capillary beds. The blood coming from the hemoconcentrator has a higher oncotic pressure than the rest of the blood in the body. As this high oncotic blood returns to the right atrium and goes to the lungs it pulls excess fluid from the lungs. This reduces pulmonary vascular resistance, improves gas exchange at the alveoli and opens the micro airways. Usually this results in a reduced right atrial preload pressure (lower CVP) along with an increased left ventricular output (increased MAP). This is explained better in the blog. If you read the blog again and pay special attention to the part about targeted capillary beds it should make better sense.
Thanks for commenting.
-Gary
The work of Woodcock & Woodcock https://linkinghub.elsevier.com/retrieve/pii/S0007-0912(17)32301-2 suggests that capillary reabsorption does not actually occur with increased capillary oncotic pressure because of the glycocalyx. Do you agree with this? If this correct then how does MUF work? I’d be really interested to know your thoughts on this!
Caroline-
Thank you for your comment.
I read this review article. As I understand it, the gist of the article is that fluid movement from the capillaries is one-way, out to the extracellular space. Reverse movement is blocked by the glycocalyx. Fluid movement out of the extracellular space is provided only by the lymphatic system. As a result, so-called volume expanders like albumin or hetastarch do not draw fluid from the extracellular space by osmosis across the capillary membrane. If this is true, it is news to me and every anesthesiologist I have ever worked with.
My early experience was with hemodialysis patients. We often used ultrafiltration to remove as much as 10 liters of fluid from them in 4 hours. That’s about 42 mls/min. Under my premise, the ultrafiltrated blood leaving the hemodialyzer has a slightly elevated oncotic pressure due to the removal of fluid. When that blood is returned to the patient’s circulation, fluid is pulled across the capillaries by the higher oncotic pressure. Under the premise of the article, the only way for the extracellular fluid to return to the circulation is by lymph flow. I don’t understand what the driving force is that causes this lymph flow to increase in volume and compensate for the 42 mls/min that is being removed. That’s the equivalent of a normal person having about 5 units of blood volume removed over 60 minutes sans the proteins and cellular components. Can lymph flow that fast? I don’t know.
My experience in heart surgery is similar. When a patient comes to the OR with excess edema, we try to remove a lot of that extra fluid. I remember the old days before we had ultrafiltrators. A 3 kg baby would arrive in surgery and immediately after surgery weigh 4 kg due to IV and prime fluids migrating to the extracellular space. The edema would make the patient look like the Pillsbury Dough Boy. Nowadays if a 4 kg baby overloaded with a liter of fluid came to surgery, I can remove that entire 1000 mls of fluid with ultrafiltration and MUF. And during MUF I would see the PIP pressure fall significantly indicating better lung compliance due to pulmonary fluid removal. This is accompanied by a fall in CVP with a concomitant increase in BP. Where else in medicine do you see a fall in CVP by half with a BP increase over ten minutes? Can forward lymph flow account for this? Can the lymph flow ‘backwards’ to make the CVP drop while increasing the BP at the same time? I think my explanation is the only one that makes sense.
I have also added 50 mls of 25% albumin to a venous reservoir during bypass and watched as my reservoir volume increased by 300 mls over the next 30 minutes. This was increased volume that I could then safely remove by UF without losing adequate circulating volume. If the increased oncotic pressure was not responsible for the fluid movement, it is difficult to believe that lymph flow added to the circulating volume in so short a period of time just after I added the albumin. Add 50 mls of 25% albumin and then remove 300 mls of fluid volume…the article did not seem to have an explanation for something like that.
In patients who go to the ICU, a sure sign they are improving is a sudden diuresis. However, this diuresis is not spontaneous and can occur from 6-48 hours later as a norm. Perhaps the delay is the result of slow lymphatic flow under these circumstances. Perhaps this is where the mechanisms described in the article work.
I am not saying that the review article and its conclusions are incorrect. They indeed may be the mechanism under certain circumstances. I am just saying there are things about this fluid movement that we don’t fully understand. But as far as clinical perfusion practice goes, it is far from the circumstances described in the review article. In the end, who am I to believe….my own eyes and experience or the article?
Hi Gary,
Love your work and the increased reach of the Krogh model in many ways thanks to you as it applies to myriad aspects of critical care physiology and more criticalists would benefit from understanding its basic tenets. That being said, there are some aspects of the glycocalyx model which is now reasonably well accepted that more readily explain the ability of some fluids to ‘draw’ volume into the vasculature. The assertion of hyperoncotic albumin and the increased fluid return to the reservoir can readily be explained by movement of fluid from the gel phase of the glycocalyx into the fluid phase of the blood, thus giving the appearance of fluid pulled from tissues without actually being able to do so. As for the mention of fluid resorption from the capillary, the model does indeed state that for non-fenestrated capillaries fluid cannot be returned to that vessel once the fluid has egressed into the tissue, but one must remember to take account of the organ as a whole, and the numerous different tissue beds which could then allow for a more ‘old school’ tissue fluid/vasculature interaction, such as the marrow, spleen etc.
Given your ability to understand OPFT, you may find wider reading around the new model can easily explain the anecdotal stories we have had told to us for so many years which we know has no real basis in fact any more!
All the best,
Tom
Tom-
Thank you for your kind remarks. I appreciate them.
I am skeptical of the ability of the glycocalyx model to explain the phenomenon associated with fluid shifts and removal during CPB. My training and experience over the 45 years that I was a perfusionist has led me to believe that two-way fluid movement across a capillary cell wall is the best explanation. I agree that there is likely resistance to reverse fluid migration within some capillary beds (the blood-brain barrier and diuretics preventing reabsorption in the kidneys are two examples). And also during periods of reduced perfused capillary density like shock when capillaries are closed to fluid transfer across their cell wall.
Many years ago in my hemodialysis days, I removed large amounts of fluid from patients in a short time (2.5 liters in 60 minutes and immediately repeated for the next three hours). I don’t think that is possible by a one way system that relies on lymph flow to restore fluid to the circulating volume. And I can’t believe that the microscopic gel layer in the glycocalyx contains that much free fluid without being replenished from the extravascular space. That is close to 4 times the total blood volume of an adult lining capillaries in a microscopic layer as fluid.
When hemoconcentrators became available for CPB and ECMO, I used them on the basis of a two-way fluid transfer. I wrote an article published in 2011 that describes these fluid movements and their consequences in pediatric CPB (see below). In that article I describe several phenomenon involving fluid transfers; the ready reservoir, the native reservoir, the effect of SIRS on fluid movement and the mechanism of third spacing.
If someone could use the glycocalyx model to explain these phenomenon using the data supplied by the article I would be impressed. For example, what I call the “ready reservoir”, you might call the glycocaylx gel phase. What is referred to as “third spacing” might be explained by the one-way fluid movement model. (Incidentally, I am skeptical that third spacing is a real phenomenon.) Capillary leakage from SIRS might be explained by a glycocalyx layer damaged by excessive fluid administration. I am open to new concepts. But until the phenomenon I see can be explained credibly I am still with the “‘old school’ tissue fluid/vasculature interaction” crowd because it seems to work.
Grist G, Whittaker C, Merrigan K, Fenton J, Worrall E, O’Brien J, Lofland G.
The Correlation Of Fluid Balance Changes On Cardiopulmonary Bypass To Mortality In Pediatric And Congenital Heart Surgery Patients. J Extra Corpor Technol, Dec, 2011;43(4):215–226
Gary
Great job Gary!
I think the folks who want to get rid of MUF should definitely read your article.
Thank you for your contribution to perfusion science.
Bharat
Bharat-
Thank you for your kind comments. One thing I have noticed about the “no need for MUF” crowd is that they seem to all have very low prime volume circuits. One way they reduce their prime volume is to remove the hemoconcentrator. That eliminates some options for them. Another thing they seem to do is cut their circuits down so that there is no slack if they need it. That eliminates other options pertaining to safety. If they are happy with their results, who am I to criticize them? But I have learned throughout the years that somethings that work at one program often do not work at another program.
good evening Garry,
how do you calculate the quantity to be ultrafiltered in an adult patient (70Kg) undergoing CEC, is there a scheme? a cordial greeting and thank you for your valuable contribution. Alcira Milan Italy
Alcira, that you for the question.
Short answer: remove between 20 and 40 mls/kg in an adult. For a 70 kg adult this would be between 1400 mls and 2800 mls total UF/MUF volume.
Longer answer: it depends on several factors.
1. Anesthesia will usually rehydrate an NPO patient with crystalloid. The people I worked with gave NPO patients 30 mls/kg = 2100 mls. So, you can remove at least 2/3rds of that; 1400+ mls
2. If you are using MUF, you will want to remove your circuit volume so that all the RBCs will be returned to the patient. With a circuit volume of 1000 mls, that should be removed. So #1 +#2 = 2400 mls.
3. If the surgeon sucks irrigation into your pump count it as volume added and remove it.
4. Blood loss should be counted as fluid removed.
5. If your patient has a diagnosis that includes congestive heart failure, they may be carrying additional fluid. A person weighing 70 kg could be carrying as much as 10 L of extra fluid due to CHF. Check to see if they have been on diuretics and assess their ankles and shins for pitting edema. If this is the case you should be able to remove an additional 2-4 L during a two-hour case.
6. If the patient comes to the OR after an aggressive resuscitation, they may have even more fluid on board. In this case, you will have to assess the situation and do your best.
Lastly, you should have a system to constantly assess the volume status. I had an excel program built into my record so that I could add and subtract fluid amounts during the case so I could make a fluid balance assessment any time during the case.
I hope this answers your questions.
Gary