The Physiology of ECPR
Extracorporeal cardiopulmonary resuscitation (ECPR) patients have a unique physiology that is radically different than the run-of-the-mill ECMO patient. Beginning within the first 5 minutes of chest compressions and manual ventilation aka cardiopulmonary resuscitation (CPR) there are changes that can have a profound impact on the post-resuscitation recovery of these patients. See Fig. 1. In normal people, the carbon dioxide (CO2) gradient between an arterial blood gas (ABG) and a right atrial venous blood gas (VBG) is less than 10 mmHg. In Fig. 1, the gradient of 8 mmHg (indicated by the green arrow) prior to cardiac arrest is located within the box outlined in red dots (1). This box looks at the CO2 gradient and the corrected anion gap (cAG) which defines the limits of a normal physiology. Once normal perfusion stops CO2 begins to build up in the tissues. Even stellar CPR cannot prevent this accumulation from happening. Within 5 minutes the pCO2 in the VBG begins increasing; a reflection of the backup of CO2 in the tissues. After 5 minutes of CPR in this example the CO2 gradient builds to 30 mmHg (indicated by the blue arrow outside the red box). This changes the intracellular pH to such a degree that protective enzymes and antioxidants are increasingly deactivated. As CPR continues the VBG pCO2 continues to build and the CO2 gradient increases until all of the protective enzymes and antioxidants are totally deactivated. Animal studies have shown that CPR for less than 15 minutes results in a brain cell pH <6.5 and pCO2 >160 mmHg. At this point the return of spontaneous circulation (ROSC) at normothermia will cause serious and often lethal damage by reperfusion injury. This is because oxygen and calcium enter the hypoxic cells faster than the protective enzymes and antioxidants can be reactivated.
The administration of sodium bicarbonate (NaHCO3) changes the arterial blood pH, but it also generates a tremendous amount of CO2 on the venous side, making the VBG even more acidotic. This is probably why NaHCO3 has never been associated with improved outcomes; making the ABG pH look better provides only false reassurance that NaHCO3 is helping.
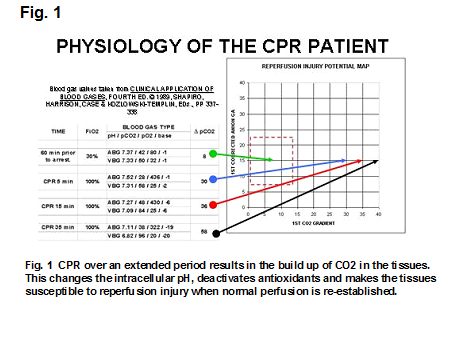
Organ systems are tolerant of low oxygen levels for a relatively long period. Even human neurons can live without oxygen for hours in cultures. However during the period of hypoxic ischemia, lactic acid accumulates (causing the cAG to increase) and CO2 builds up to such a degree that all of the protective enzymes and antioxidants within the living cell are deactivated. When perfusion is suddenly restored there is an influx of oxygen and calcium. Oxygen and calcium are the two primary mediators of reperfusion injury, causing oxygen stress and calcium stress. If they flow into the ischemic cells where protective enzymes and antioxidants have been deactivated, there is uncontrolled tissue damage. In Fig 1, reperfusing this patient with normothermic ECMO after 35 minutes of CPR would probably cause lethal reperfusion injury damage.
ECPR strategy addresses the reperfusion issue at several levels. First it cools the patient. Hypothermia is the only thing known to prevent reperfusion injury from oxygen stress. Nobody really knows why. Here is my educated guess. Hypothermia is commonly used in varying degrees during cardiopulmonary bypass (CPB) in open heart surgery. Deep hypothermic circulatory arrest (DHCA) is a well proven technique used when the surgery being performed requires all perfusion to be stopped. The patient is cooled to 18°C to reduce the metabolism; about 1/10th of normal (the brain metabolism only drops to 1/5th of normal at 18°C) (2,3) at which point the pump is stopped and the patient’s blood is drained out. The patient can be safely maintained in this state of pseudo-death for up to 45 minutes. During this time period the patient’s tissues still gradually become acidotic due to accumulated lactic acid and CO2 even though the metabolism is greatly reduced (4). Being totally arrested at 18°C for 45 minutes is like being totally arrested without CPR for 4.5 minutes (9 minutes for the brain) at normothermia. After 45 minutes of DHCA and just after the pump is restarted, the first VBG can look as bad as this; pvH = 6.90, pvCO2 = 110, pvO2 = 20, Base = -10 (after temperature correction). Compare this to the patient’s VBG after 35 minutes of CPR at normothermia in Fig. 1; pvH = 6.82, pvCO2 = 96, pvO2 = 20, Base = -20. At the end of the DHCA period, the patient is placed back on CPB with full perfusion without harm even though the protective enzymes and antioxidants are deactivated due to both the low tissue pH and the low temperature. As the patient is gradually rewarmed, the excess intracellular CO2 is removed and the intracellular pH returned to normal; reactivating the protective enzymes and antioxidants.
So why is it that a very cold, acidotic patient can be reoxygenated without causing a lethal reperfusion injury? I think the answer lies in the biothermal chemistry of oxygen. One published study demonstrated a 75% reduction in leukocyte reactive oxygen species (ROS) production when the patients’ core temperatures were reduced by just 4°C (5).Oxygen is more reactive at warmer temperatures within biologic systems. So if the temperature is low, not as many ROS form. ROS are composed of deadly poisons such as ozone, superoxide radicals and peroxides. In warm blooded animals excess oxygen entering the body converts to ROS. To combat this, evolution has produced an abundance of antioxidants to counter act the ROS threat. Other animals are cooler, so the oxygen entering their bodies tends to form fewer ROS. Cold blooded reptiles that have no natural predators like crocodilians and tortoises and fish like sturgeons and blue catfish can live well over 100 years and grow to tremendous size. Fewer ROS in their cold bodies is probably one of the reasons why their organ systems don’t wear out as fast as mammals.
When reperfusion starts, the cool temperature protects the acidotic DHCA patient against reperfusion injury until the perfusionist corrects the VBG and intracellular pH by blowing off the excess tissue CO2 as rewarming progresses. Correcting the pH reactivates the protective enzymes and antioxidants. Likewise, by cooling the ECPR patient the ROS that can form during reperfusion are reduced. This gives more time to correct the tissue pH and reactivate the antioxidants. Ventilating the oxygenator with room air minimizes the infusion of excess oxygen that can potentially form more ROS.
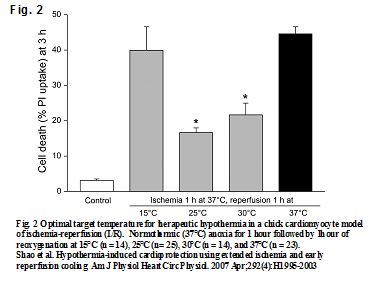
In some programs, CPR patients are arbitrarily cooled topically or by cold intravenous fluid to no less than 32°C. The reason being that if ROSC is achieved a heart that is too cold may not beat rigorously enough to support the patient’s perfusion needs. A big advantage of ECPR is that the heart need not support the patient’s circulation, that being done by the pump instead. So the option of using lower temperatures is possible. But nobody really knows what the best hypothermic temperature is for resuscitation to prevent reperfusion injury. One bench top study shows that after 1 hour of normothermic anoxia, cardiac cells can be reoxygenated with the least amount of damage when the tissues are first cooled to 25°C. See Fig. 2. (6). Future experience will most likely show that the longer the patient is arrested at normothermia the deeper he will need to be cooled for a safe rescue.
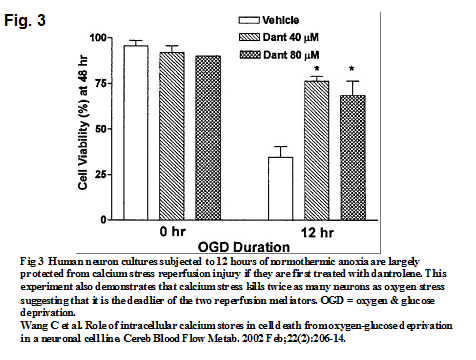
The second strategy is to prevent calcium stress which is even more dangerous than oxygen stress. Dantrolene is FDA approved for treatment of spastic muscle spasms and variants of malignant hyperthermia (MH). Its mechanism of action is to bind to the ryanodine receptors on mitochondria and decrease free intracellular calcium concentration. These properties can prevent the calcium from damaging the mitochondria in the ischemic brain and heart. A study by Wang demonstrates that neuronal cultures exposed to simulated ischemia for many hours can be protected from calcium stress when treated with dantrolene (7). See Fig. 3. A secondary finding is that calcium stress (which is prevented by dantrolene) kills twice as many cells as oxygen stress (which is not prevented by dantrolene). This means that calcium stress is probably much deadlier than oxygen stress. A calcium free prime should be used for the ECMO pump to prevent the infusion of any additional calcium in ECPR. Dantrolene should also be added to the pump prime.
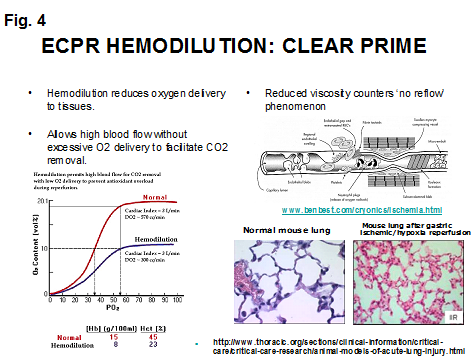
Lastly a clear pump prime is used to hemodilute the patient. One of the dangers of reperfusion injury is the clumping of activated white blood cells (WBC) and platelets in vital capillaries, particularly in the lungs. Physiologists call this ‘no reflow phenomenon’; physicians call it disseminated intravascular coagulation (DIC) (8). Hemodilution helps to prevent this and makes it easier to pump blood through the clogged capillaries, particularly in the lungs (9). See Fig. 4. Hemodilution also reduces the amount of oxygen infused into the patient while still allowing for high pump flows to remove excess intracellular CO2.
Hemodilution is the antithesis of early goal directed therapy (EGDT) which is meant to maximize oxygen delivery (10) . EGDT may even be dangerous in the patient at risk for reperfusion injury (11). During ECPR, hypothermia makes hemodilution safe to use because the oxygen requirements are greatly reduced.
It is interesting that the treatment for MH (particularly those patients who have cardiac arrest) is very similar to this ECPR strategy. In the MH algorithm, dantrolene is given immediately. Because it has so few side effects dantrolene is safe to give prophylactically in most suspect patients receiving gas anesthesia or nondepolarizing neuromuscular blocking agents and will not interfere with resuscitation if cardiac arrest should occur in the MH patient (12,13,14,15). This is followed by the infusion of cold normal saline (NS) which does several things. It cools the patient to counter-act any hyperthermia that occurs. Since potassium and myoglobin are commonly released in MH patients, the NS also acts to hemodilute the blood potassium which may cause the cardiac arrest and decreases the myoglobin concentration in the blood which can clog vital capillaries, particularly in the kidneys. Hemodilution also reduces the amount of oxygen sent to the MH patient’s ischemic brain once ROSC is achieved. Anecdotal case reports describe MH patients undergoing CPR for extended periods (over 60 minutes) and still surviving neurologically intact. Some MH patients who did not achieve ROSC required the use of mechanical circulatory support. It is not just happenstance that CPR resuscitation is frequently successful in MH patients because the MH strategy, in large part, emulates the steps needed to protect a generic patient undergoing CPR for an extended period who requires ECPR.
The advanced life support (ALS) and pediatric advanced life support (PALS) algorithms are etched in stone. But if I had my ‘druthers’, CPR patients who evolve into ECPR patients would have the following algorithm:
- After the patient has been arrested for 5 minutes a dose of dantrolene should be given. If ROSC is achieved, dantrolene may help to protect the brain from calcium stress. (It is my opinion as a perfusionist that the first drug any cardiac arrest patient should receive is dantrolene.)
- After 10 minutes of CPR, the ECPR team should be activated and the IV infusion of calcium free, ice cold crystalloid should begin (30 mls/kg). This fluid should be given preferentially as close to the head as possible to achieve a more effective core cooling. If the fluid is given below the waist, its ability to cool the brain will be diminished by passing through warm non-essential body tissues before reaching the heart.
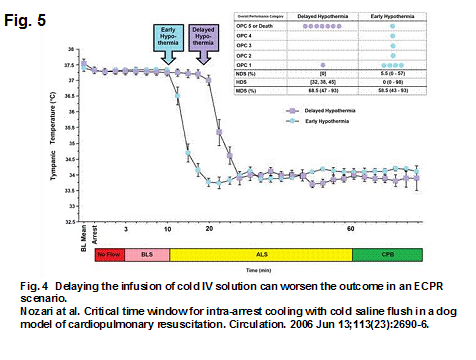
An experiment in dogs using an ECPR scenario by Nozari in 2006 demonstrated that a delay of even 10 minutes in administering cold IV solution led to much worse outcomes (16). See Fig. 5.
- After 20 minutes of CPR, ROSC becomes less likely. At this point no additional epinephrine, calcium or bicarbonate should be given. Emphasis should be placed on quality CPR and positioning the patient for cannulation.
- ECPR should be instituted with calcium free crystalloid prime with a dose of dantrolene, hypothermia and hemodilution. An evaluation of the VBG after ECPR is initiated to determine the state of tissue acidosis can guide the perfusionist in correcting the tissue pH and reactivating the protective enzymes and antioxidants before rewarming.
ECPR Case: A 38 week gestation male with transposition of the great arteries, cleft palate, and hemivertebrae underwent an arterial switch operation at two weeks of life. The patient was doing well when, at three days after heart surgery, there was an unexpected cardiovascular collapse due to junctional ectopic tachycardia (JET). Soon after the initiation of the code, ECPR was activated and ice was placed on the patient’s head. During the 90 minutes of CPR, good perfusion was maintained with good near infrared spectroscopy (NIRS) monitor levels. The arterial blood gases (ABGs) were good under the circumstances. No venous blood gases (VBGs) were taken during CPR.
The patient was placed on VA neck ECMO while chest compressions were ongoing. The ECMO run lasted 122 hours (5 days) without any significant complications. During the five days on ECMO the patient was treated pharmacologically for JET and observed closely for reassurance that it would not resume. Renal output was good throughout the run. The patient did have some seizure activity following the ECMO run. A brain CT scan showed a left middle cerebral artery distribution infarct to the left frontal and parietal lobes. A left sided infarction might seem strange for two reasons.
First, the infarct was on the left side which is the side opposite of the cannulation side where the right carotid artery and its blood flow to the brain was completely ligated. Most infarctions in VA neck cannulation ECMO patients occur solely on the left side (61%) with only 11% occurring solely on the right side, according to my in-house ECMO data. The remaining 28% of infarcts occur bilaterally. This is probably because a hypoxic and ischemic patient like this is at risk for reperfusion injury to the brain once reoxygenation and recalcification by the ECMO pump is started. Ligation of the right carotid probably paradoxically protects the right brain from immediate reperfusion by limiting the rapid reoxygenation from the ECMO pump.
The second reason is that this patient underwent CPR for 90 minutes. The brain damage incurred seems minimal for such a long period of CPR. Core cooling quickly with the ECMO pump using a calcium free clear prime with Dantrolene affords the maximum protection that I know of from reperfusion injury.
The patient was eventually extubated and discharged alive and did well thereafter. This is a good example of everything working well together. Although it would have been nice to have a shorter CPR period, everything else seemed to work seamlessly. Furthermore, it holds the promise that resuscitation teams can get better at ECPR, eventually avoiding organ damage altogether in the future, at least in cardiac patients.

ECMO oxygenators are not really the best design but simply a regular MO with different fibers. The heat exchanger should be stainless steel at a lower surface area because we only want to maintain temperature. The Oxygenator fibers are ok but the design needs to be different. Here you simply have a MO with half the guts simply replaced. Pretty sad.
The best design would be Ron Leonard’s Sarns SMO. Tandem copied 1/2 of the Ron’s design but too little m2. They would be better to go to Terumo and ask for the moldings and just replace the fibers.
Too bad the FDA makes it difficult to make the companies more flexible.
Thanks, Jorge. Good thoughts and insight into a difficult situation.. I wonder why the FDA would rather we use an “approved” oxygenator known to fall apart during use rather than an “unapproved” oxygenator with a extensive and proven track record overseas?
Read Dr. Somer’s powerpoint. Ron Leonard from Sarns in the late 80’s was a great engineer. I am sure Tandem read this article as well.
I still like bags but they are no longer available in pediatrics. Bummer.
http://scansect.org/wp-content/uploads/2015/04/Pressure.pdf
Similar to the concept of elective cardiopulmonary bypass, used in open heart surgery, oxygenation and perfusion can be maintained with an ECMO device in patients undergoing cardiovascular collapse.
Gary,
Great reading. Why has this not caught on. What was your protocol in KC?
Chuck- Sometimes it takes a while before people adopt new ideas.
The protocol at my old hospital was like the article says. We primed with a calcium free, clear crystalloid to which an appropriate dose of dantrolene was added. We left the prime at room temp and went on pump to quick cool the patient to 32C (hemodilution, hypocalcemia and hypothermia). We used a room air sweep gas to keep the paO2 at about 90-100 mmHg. We monitored the p(v-a)CO2 with the goal of reducing it to 10 mmHg or less. We kept the pump flow high (2.5 L/min/M2, if possible) and the sweep gas high. But we were often unable to achieve good blood flow for several hours due to the excessive use of epi during CPR. I tried to get the intensivists to limit their epi, Ca and bicarb to a single dose before calling for ECPR, but many of them wouldn’t. Once we got the p(v-a)CO2 down to 10 mmHg we started to UF or added PRBC and increased the sweep gas FiO2 to 40%. If the patient was still acidotic we gave some bicarb but were careful never to increase the osmolarity beyond 300 mosmoles/L. We usually stayed cool 12-24 hours. Once the heart was beating and the pH normalized we would give some Ca gluconate if the calcium was low. Then we would rewarm over 6 hours and leave the patient on ECMO if needed.
I have been retired for 3 years and there have been a lot of changes at my old program. So I don’t know what they do now.
-Gary
good afternoon, first of all congratulations for your blog, very educational. I am a perfusion student in Spain and I am doing research work on the different management protocols for ECPR. I would like to share them with me if it were possible and available. I would really appreciate it, my email is: miguel.andresgasco@hotmail.com
Thank you so much for everything
Miguel
Thank you for your comments. I will send you this article: Grist G. Extracorporeal membrane oxygenation (ECMO) or extracorporeal cardiopulmonary resuscitation (ECPR): A critical life or death choice. Progress in Pediatric Cardiology; 2008 January; 24(2):113-116.
To truly understand ECPR you need to understand reperfusion injury. The section on oxygen pressure field theory (OPFT) on this web site is very informative about the mechanisms of reperfusion injury. You can download the OPFT article PDF here : https://perfusiontheory.com/oxygen-pressure-field-theory/
Best wishes on completing your education and pursuing a career as a perfusionist.
Gary