Blood and Fava Beans (glucose-6-phosphate dehydrogenase (G6PD) deficiency)
No, this is not an article about the favorite side dish of a bloodthirsty murderer (ala Hannibal Lecter of “The Silence of the Lambs” fame). It is about broad beans, blood and a rare perfusion complication. Favism, named after the fava bean, is a hereditary disease that can cause significant levels of hemolysis. Hemolysis during extracorporeal support of all types is associated with increased systemic and pulmonary vascular resistance, an altered coagulation profile, platelet dysfunction, renal tubular damage, infection and increased mortality (1). Hemolysis can occur as a result of mechanical damage to the RBCs by the pump, oxygenator, tubing and other components of the circuit as well as an air interface. However, sometimes an idiopathic hemolysis can occur. On occasion I have seen post CPB patients with greater than normal hemoglobinuria; suggestive of a hemolytic transfusion reaction even though the patient did not receive a transfusion. So, I blamed the hemolysis on the pump. If they did get RBCs on CPB, the direct Coombs test was invariably negative in these patients. I have also seen unexplained hemolysis in ECMO patients that even changing the circuit could not reverse.
One possible cause of this mysterious hemolysis might be a glucose-6-phosphate dehydrogenase (G6PD) deficiency (G6PDD). Severe cases are known as favism because the consumption of broad beans can trigger a severe hemolytic episode in people who have a severe deficiency of this enzyme. The G6PD gene is located on the X chromosome. Thus the deficiency state is a sex-linked trait seen only in hemizygotic males. Most female carriers are asymptomatic. An estimated, 30,000 to 40,000 black male G6PDD babies, a high risk group for favism, are born annually in the United States (2).
G6PD catalyzes the pentose phosphate pathway within red blood cells which eventually leads to the creation of glutathione, an antioxidant. G6PDD red blood cells lack adequate levels of this antioxidant. This makes red cells susceptible to oxidative stress which can lead to cell rupture, hemolysis and even anemia. It seems ironic that the only cell capable of carrying oxygen in the body may be susceptible to the toxic effects of oxygen.
The hemolysis usually has a trigger such as certain foods, drugs, stressors or infection and older red cells are affected most because of their fragility. The hemolytic crisis may occur within hours of exposure to the trigger. Severe variants can cause hemolytic anemia even in the absence of an obvious trigger. In severe cases, hemoglobinuria and peripheral circulatory collapse can occur. There may be a rapid drop in hematocrit which is concomitant with a rise in plasma hemoglobin and unconjugated bilirubin. Neonatal jaundice may also occur in G6PDD infants (3).
G6PDD is the most common enzyme defect in humans with more than 400 million cases world wide. The highest G6PDD frequency is 70% among Kurdish Jews, 10% among Africans, black Americans, and populations of Mediterranean and South East Asian countries, but only 0.05% in middle and northern European populations (4). Some G6PDD variants are associated with a resistance to malaria which might explain its naturally selected increased prevalence in tropical and sub-tropical populations(3). However, G6PDD is no longer localized; it has become a worldwide disease (5).
The World Health Organization classifies G6PDD variants according to the magnitude of the enzyme deficiency and the severity of hemolysis (Table 1). Class I patients have a severe deficiency and chronic hemolytic anemia. Class II patients also have severe deficiency with periodic hemolysis. Class III patients have moderate deficiency with intermittent hemolysis associated with a stressor such as infection or drugs. Class IV patients have no deficiency or hemolysis. Class V variants have increased enzyme activity. Classes IV and V are not considered to be clinically significant.
G6PDD patients who undergo cardiopulmonary bypass for cardiac surgery have an increased postoperative ventilation time, lower postoperative oxygen levels, more hemolysis and a greater need for blood transfusion (6). G6PD, working in concert with other factors, is thought to markedly influence hypoxic pulmonary reactivity (7). Therefore, G6PDD patients might be at a greater risk for pulmonary hypertension which could explain their decreased pulmonary function in the post CPB period.
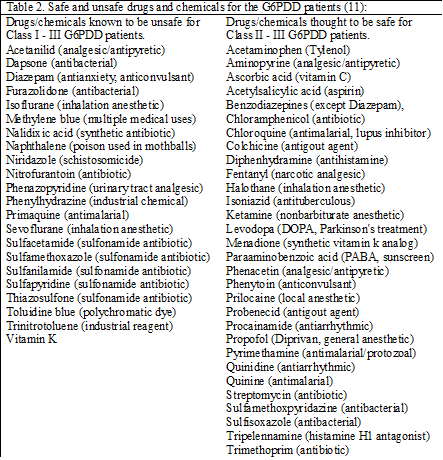
Common perioperative drugs associated with G6PDD hemolysis include sevoflurane, isoflurane, diazepam and vitamin K. Other drugs are also known to precipitate hemolysis in G6PDD patients. In contrast, some drugs are known to be safe. See Table 2. A more complete list can be found at <http://www.g6pd.org/favism/>, the Favism Association web site. Any drug that causes methemoglobinemia can precipitate hemolysis in G6PDD patients (8,9). Nitric oxide gas in concentrations greater than 8 ppm is known to cause methemoglobinemia in neonates (10). However, there are no studies that examine the effect of nitric oxide gas on G6PDD neonates.
The most common precipitant of G6PDD hemolysis is infection (11). However, other perioperative factors can precipitate a G6PDD hemolytic episode, such as acidosis, hyperglycemia, and hypothermia less than 32C.
Blood transfusion recipients have developed hemolysis as a result of receiving G6PDD donor blood (12,13). It is possible that this could be confused with a transfusion reaction. The direct Coombs test is positive if hemolysis is caused by an immune process. However, because hemolysis in G6PDD is not an immune process, the direct Coombs result is negative. CPB can certainly be a stressor that triggers hemolysis either in a G6PDD cardiac surgery patient or in the donor blood used to prime the pump. Bone marrow transplant recipients have contracted G6PDD from affected donors (14,15). Neonates transfused with G6PDD donor blood may have a smaller drop in total serum bilirubin after transfusion. They may require a repeat exchange transfusion as well as a longer duration of phototherapy (21).
The frequency of G6PDD donor blood units is probably reflected in the frequency of G6PDD in the population. Some examples include Spain (0%), India (2%), China (5%), Africa (10%) and Iran (15%) (14,17-19). G6PDD screens are typically not performed on donor blood, so it is possible for patients to unknowingly receive G6PDD blood. The frequency of G6PDD donors in the United States at large has not been reported. However, the prevalence of G6PDD in the combined populations of African, Asian, and southern Mediterranean peoples living in the northwest USA is 11.6% (20).
The American Red Cross may specifically reject patients diagnosed with favism. However, most local blood banks do not specifically ask if a donor has favism or G6PDD. Such an inquiry would probably be ineffective because most people with G6PDD are not aware that they have the condition. G6PDD donor blood is accepted and transfused because blood banks in the USA do not routinely screen for G6PDD. The general consensus in the USA is that G6PDD donor blood is safe to transfuse even in neonates (2).
There are no published studies of extracorporeal support patients with G6PDD or patients who were recipients of G6PDD donor blood while on mechanical support, just a few case reports. According to the ELSO reports, the frequency of hemolysis in ECMO patients is about 10%. The possibility exists that some of this hemolysis could be from G6PDD patients or G6PDD donor blood. If a CPB, IABP, VAD or ECMO patient develops an idiopathic hemolysis, there is a laboratory test for the G6PD level. The test is most frequently ordered by a Hematology Service as part of a work up for anemia, hemolysis or high risk patients, but it can be used to eliminate G6PDD as a cause for hemolysis in extracorporeal support patients. “G6PD” is a quantitative test reported in units of activity. The test may not discern between a patient with G6PDD or transfused G6PDD donor blood and may be negative during a hemolytic crisis when the older and defective RBCs are replaced by younger cells. In such cases, the test has to be repeated. So, the next time a case of unexpected hemolysis occurs, check for G6PDD before blaming the pump.