The Bicarbonate Lag Phenomenon in Neonates
—– Original Message —–
Dear Gary-
My chief of surgery indicated to me a couple of weeks ago that we have had some issues in the ICU with a couple of neonates having a significant metabolic alkalosis that persisted for days, of course accompanied by significantly elevated compensatory PCO2’s that resulted in prolonged vent times. This condition has on a couple of circumstances, persisted for 3-4 days before the babies could be extubated.
They are questioning [blaming] this condition on intraoperative administration of Bicarb by the perfusion staff. We typically treat negative B.E. once they reach around -5 or more with “normal” PCO2’s.
I reviewed one pump record of a patient who experienced the described ICU course, and his last ABG in the OR showed a normal PCO2, normal pH, normal HCO3… We still do cases with sometimes multiple periods of circ arrest and cerebral perfusion, and often cool to 18 degrees with frequent periods of low flow [1.0 index or less].
Have any of you experienced this phenomena? Any thoughts or suggestions? Do patients “sequester” Bicarb in some fashion or is there some sort of “rebound” effect?
Any feedback or insights would be appreciated…and thanks in advance!!!
-name withheld
My response:
What you are describing is what I call the ‘bicarbonate lag phenomenon’. I assume that you are initially priming with Plasmalyte or some similar crystalloid. Then you add blood and bicarbonate to balance the pH. Plasmalyte contains no bicarbonate, but it does have acetate and gluconate and lactated Ringer’s solution (LR) contains lactate. When you give Plasmalyte or LR to an adult, the acetate and gluconate (or lactate) will convert to bicarbonate within a few minutes after passing thru the patient’s liver. That is why you can give a ton of this type of crystalloid to an adult without diluting the circulating bicarbonate. If you just give normal saline (NS) solution to an adult, he will soon become acidotic just due to the bicarbonate dilution.
Even though you may remove some of the Plasmalyte crystalloid by ultrafiltration after adding the blood, there is still some acetate and gluconate (or lactate) left in the prime. Then you add the sodium (Na) bicarbonate (HCO3) to normalize the prime pH. Two things happen; 1) by adding the NaHCO3 you increase the prime Na to fairly high levels which may be within the normal range, but may exceed the infant’s baseline Na. This will force the infant’s Na up during CPB and somewhat impair renal function in the post-op period, delaying diuresis. In the normal person, if the Na increases above the usual level, the kidney’s think the body is dehydrated and try to conserve water by reducing urine output. Secondly, the acetate and gluconate (or lactate) left in the patient will finally convert to bicarbonate after about 6-10 hours post-op because an infant’s liver cannot quickly convert these to HCO3. Even though the HCO3 was normal in the OR, it will rise in the post-op period due to this late acetate/gluconate/lactate conversion. As this conversion progresses and the renal function is impaired by relatively high serum Na, the excess HCO3 is not efficiently removed. The retained HCO3 causes the metabolic alkalosis; i.e. bicarbonate lag phenomenon.
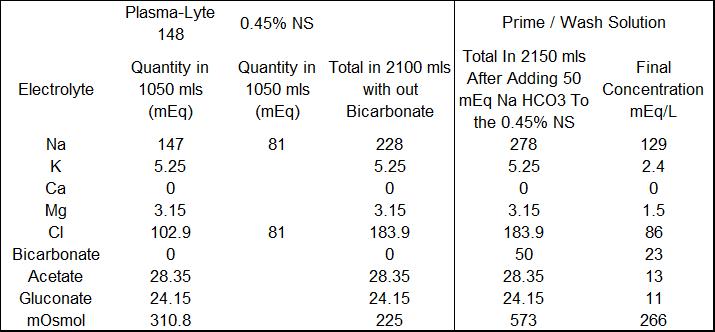
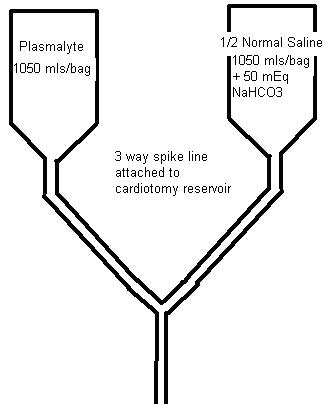
Our solution to this problem is to make a crystalloid prime/wash solution more compatible with the neonate’s physiology and renal function. We take a bag of Plasmalyte and a bag of 0.45% NS to which 50 mEq of NaHCO3 has been added and put them on a dual spike. Then we run equal volumes of each solution into the circuit to prime it and/or to wash any blood we add to the prime, removing the excess fluid by UF. The combined solutions have a lower limit osmolarity (266 mosmoles/L) and Na (129 mEq/L) concentration. During CPB, NaHCO3 can be safely added without raising the Na excessively. Any extra prime crystalloid can be added for volume during CPB without giving the infant an excess dose of acetate and gluconate as would happen if you gave straight Plasmalyte. I have attached a table showing the prime constituents and their final concentration as we mix them. We have been doing something like this for many years. The result has been less post-op edema, better diuresis and very little alkalosis in the post-op period.
That’s my interpretation of your situation. If you find the metabolic alkalosis to be caused by something else, I would be interested in knowing the cause.
Good luck-
-Gary Grist