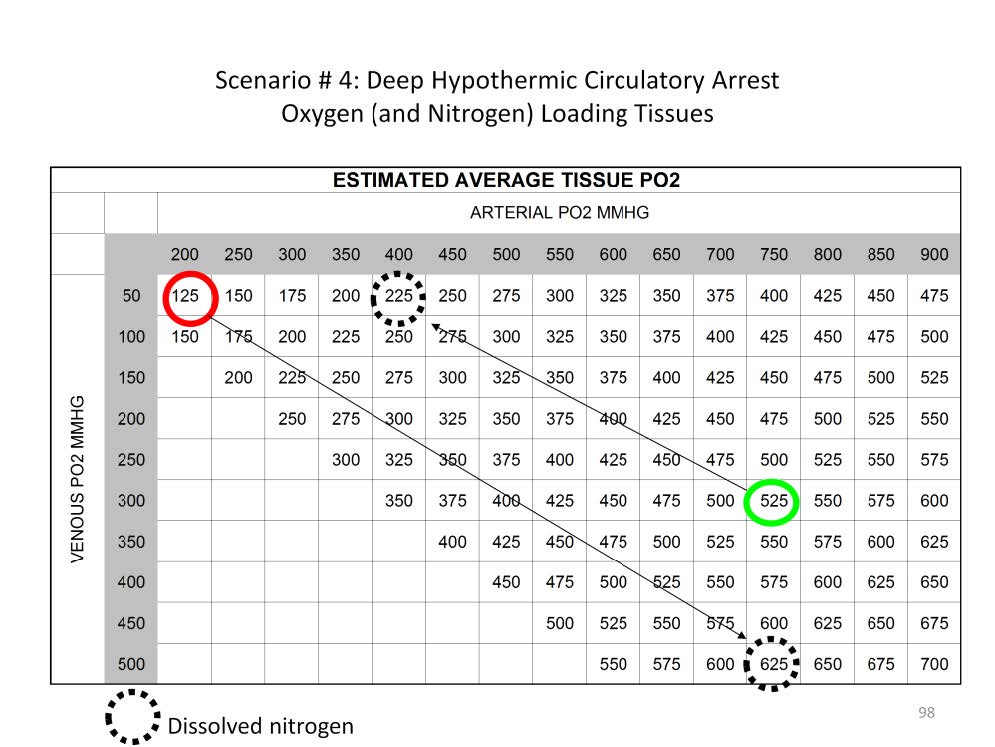
The solubility of oxygen in saline increases from 0.03 ml/mmHg/L to 0.042 ml/mmHg/L as the body temperature drops from 37C to 18C, an increased dissolved content of 40%. Over the same temperature range overall oxygen consumption drops to 10% of normal (20% in the brain). So prior to profound hypothermic arrest the amount of dissolved oxygen in the tissues can be increased substantially by increasing the paO2 of the blood coming from the oxygenator. Since the hemoglobin is essentially non-functional at this temperature, an estimate of the dissolved tissue oxygen can be derived by averaging the paO2 and the pvO2.
For example, at 18C a paO2 of 200 mmHg and a pvO2 of 50 mmHg the average tissue pO2 would be 125 mmHg: (200 mmHg + 50 mmHg) / 2 = 125 mmHg. Correspondently the amount of nitrogen dissolved in the tissues would be an average of 625 mmHg.
Alternatively at 18C a paO2 of 750 mmHg and a pvO2 of 300 mmHg the average tissue pO2 would be 525 mmHg: (750 mmHg + 300 mmHg) / 2 = 525 mmHg. But the nitrogen dissolved in the tissues would only be 225 mmHg.
This means that over four times as much oxygen can be dissolved in the brain at 18C if a paO2 of 750 mmHg is infused and the pvO2 reaches 300 mmHg. Targeting a pvO2 of 300+ mmHg assures that the tissues are well loaded with dissolved oxygen prior to circulatory arrest.
If the perfusionist chooses not to load oxygen by using a lower FiO2, then, by default, s/he is loading nitrogen into the cold tissues. Like oxygen, nitrogen is more soluble at lower temperatures. During rewarming, nitrogen (like oxygen) will tend to come out of solution and form bubbles in the blood vessels, stroma or even the parenchyma of the organs. Because nitrogen is an inert gas and will not be consumed by the living tissues, nitrogen bubbles can block or compress vital capillaries for extended periods.

Perfusion Theory is an educational platform for the Oxygen Pressure Field Theory (OPFT). August Krogh’s theoretical concept of the oxygen pressure field is explained and then applied to clinical applications in perfusion practice.